Gujarat Board GSEB Textbook Solutions Class 8 Science Chapter 6 Combustion and Flame Textbook Questions and Answers, Notes Pdf.
Gujarat Board Textbook Solutions Class 8 Science Chapter 6 Combustion and Flame
Gujarat Board Class 8 Science Combustion and Flame Textbook Questions and Answers
![]()
Question 1.
List conditions under which combustion can take place.
Answer:
Conditions necessary for combustion are:
- Presence of a combustible substance.
- Attainment of ignition temperature.
- Proper supply of air to provide oxygen.
Question 2.
Fill in the blanks.
(a) Burning of wood and coal causes _______ of air.
(b) A liquid fuel, used in homes is _______.
(c) Fuel must be heated to its before _______ it starts burning.
(d) Fire produced by oil cannot be controlled by _______
Answer:
(a) pollution
(b) kerosene.
(c) ignition temperature
(d) water.
![]()
Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
The use of CNG in place of petrol and diesel reduce pollution in following ways:
- It produces less carbon monoxide gas.
- It produces less carbon dioxide gas.
- It produces less amount of sulphur dioxide and nitrogen dioxide which cause acid rain.
- No residue remains after combustion.
Question 4.
Compare LPG and wood as fuels.
Answer:
Differences:
LPG:
- It is a gaseous fuel.
- It does not produce smoke.
- Its calorific value is more (55000 kJ/kg).
- It is easily stored in cylinders.
- It does not cause any pollution.
Wood:
- It is a solid fuel.
- It produces smoke.
- Its calorific value is less (17000 kJ/kg).
- It requires more space to store.
- It causes pollution.
![]()
Question 5.
Give reasons:
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
(a) Water is a good conductor of electricity. It conducts electricity and may result electric shock.
(b) LPG has more calorific value and produces no pollution. So it is better domestic fuel than wood.
(c) The ignition temperature of paper is less, so it catches fire easily. It does not catch fire when wrapped around aluminium pipe because aluminium absorbs the heat, so paper does not attain its ignition temperature.
Question 6.
Make a labelled diagram of a candle flame.
Answer:
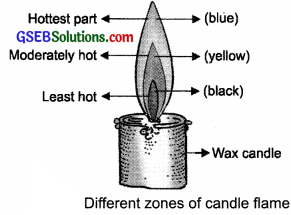
Question 7.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
kilojoules per kg (kJ/kg)
Question 8.
Explain: how CO2 is able to control fires.
Answer:
- CO2 forms a blanket around fire due to which supply of air is stopped.
- CO2 also brings down the temperature of the fuel.
Question 9.
It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
The green leaves contain some water due to which the ignition temperature of leaves increases and they do not catch fire easily while dry leaves have no water, so they catch fire easily.
Question 10.
Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
A goldsmith uses the outer zone (non- luminous zone) of a candle flame to melt gold and silver because it is the hottest zone and has more temperature.
![]()
Question 11.
In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answer:
Total mass of fuel = 4.5 kg
Total heat produced = 180,000 kJ
Heat produced by burning 1 kg of fuel
= 180,000 kJ/4.5 kg = 40,000 kJ/kg.
So, calorific value of fuel = 40,000 kJ/kg.
Question 12.
Can the process of rusting be called combustion? Discuss.
Answer:
The process of rusting cannot be called combustion because in this process no heat and light is produced. Due to this reason iron is not considered as combustible substance.
Question 13.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer:
The water heated by Ramesh will get heated in a shorter time because he kept his beaker near the hottest zone of the flame.