Gujarat Board GSEB Textbook Solutions Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids Textbook Questions and Answers, Additional Important Questions, Notes Pdf.
Gujarat Board Textbook Solutions Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
GSEB Class 12 Chemistry Aldehydes, Ketones and Carboxylic Acids InText Questions and Answers
![]()
Question 1.
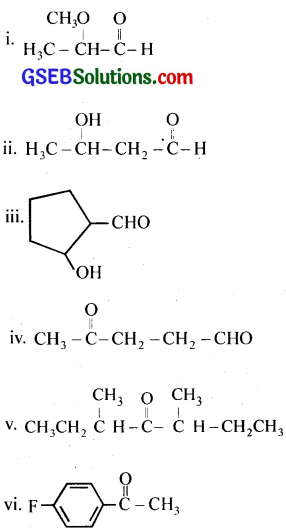
Write the structures of the following compounds.
(i) α -Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec. butyl ketone
(vi) 4-Fluoroacetophenone
Answer:
Question 2.
Write the structures of products of the following reactions.
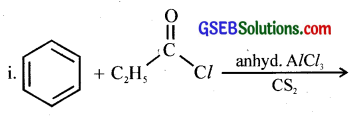
ii. (C6H5CH2)2Cd + 2CH3COCl →
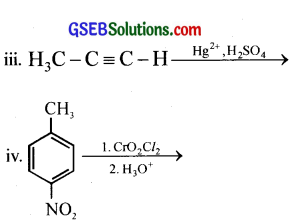
Answer:
![]()
Question 3.
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Answer:
CH3CH2CH3 < CH3OCH3 < CH3CHO < CH3CH2OH.
This order can be predicted on the basis of intermolecular force operating between them, these are having comparable molecular mass. CH3CH2OH undergoes the strongest H- bonding. In CH3OCH3 and CH3CHO dipole-dipole attraction is more in CH3CHO, since CH3CHO is more polar than CH3OCH3 therefore its boiling point is more than CH3—O—CH3. Propane being non-polar therefore, weak van der Waals’ forces exist between them.
Question 4.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
Answer:
(i) The electron-withdrawing inductive effect, as well as steric hindrance, is in the order: Butanone > Propanone > Propanal > Ethanal. Thus, the reactivity in nucleophilic addition reactions decreases in the order, Butanone < Propanone < Propanal < Ethanal.
(ii) Acetophenone is a ketone and all the others are aldehydes. Thus, acetophenone is the least reactive. The – CH3 group in p-tolualdehyde is electron-donating while the – NO2 group in p-nitrobenzaldehyde is electron-withdrawing. Thus, p-tolualdehyde is less reactive and p-nitrobenzaldehyde is more reactive than benzaldehyde. Thus, the reactivity is in the order, Acetophenone < p-tolualdehyde < benzaldehyde < p-nitrobenzaldehyde.
![]()
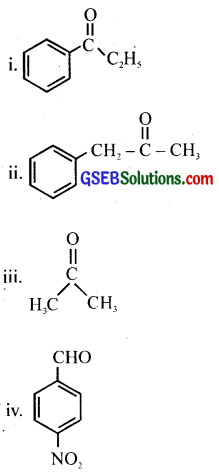
Question 5.
Predict the products of the following reactions:
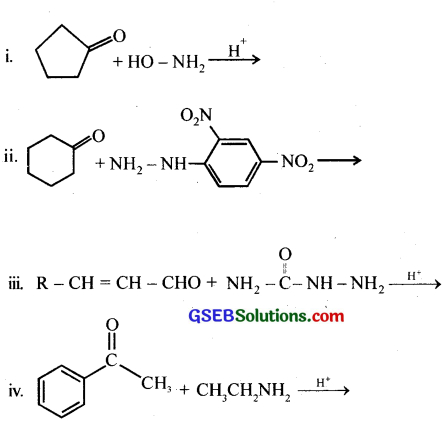
Answer:
Question 6.
Give the IUPAC names of the following compounds:
(i) Ph CH2CH2COOH
(ii) (CH3)2C = CHCOOH
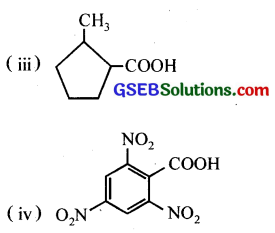
Answer:
(i) 3-Phenylpropanoic acid
(ii) 3Methylbut-2-enoic acid
(iii) 2-Methylcyclopentanecarboxylic acid
(iv) 2,4,6-Trinitrobenzoic acid
Question 7.
Show how each of the following compounds can be converted to benzoic acid,
(i) Ethylbenzene
(ii) Acetophenone
(iii) Bromobenzene
(iv) Phenylethene (Styrene)
Answer:
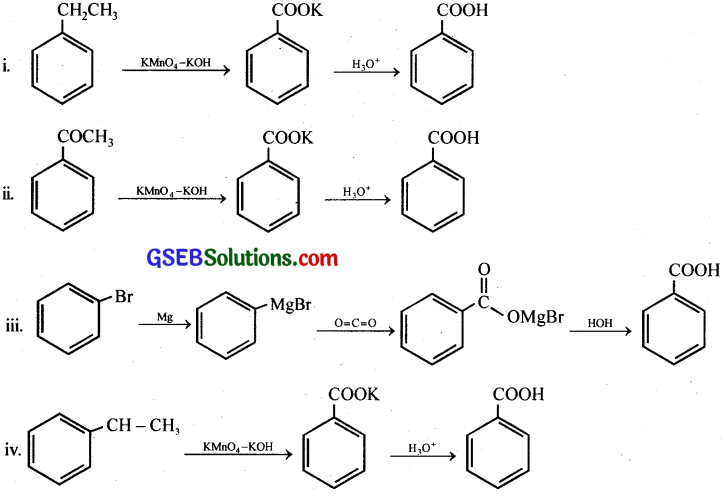
Question 8.
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CH3FCH2CO2H
(iv) 
Answer:
(i) FCH2COOH (due to -I effect of F)
(ii) FCH2CO2H (due to much stronger -I effect of F over Cl)
(iii) CH3CHFCH2COOH (Inductive effect decreases with distance i. e. 3-fluorobutanoic acid is stronger than 4-fluorobutanoic acid)
(iv) 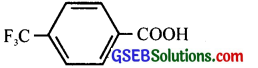
(F is electron-withdrawing)
GSEB Class 12 Chemistry Aldehydes, Ketones and Carboxylic Acids Text Book Questions and Answers
Question 1.
Name the following compounds according to the IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH3CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH = CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
Answer:
(i) 4-Methylpentanal
(ii) 6-Chloro-4-ethylhexan-3-one
(iii) But-2-enal
(iv) Pentane-2,4-dione
(v) 3,3,5-Trimethylhexan-2-one
(vi) 3,3-Dimethylbutanoic acid
(vii) Benzene -1,4-dicarbaldehyde
![]()
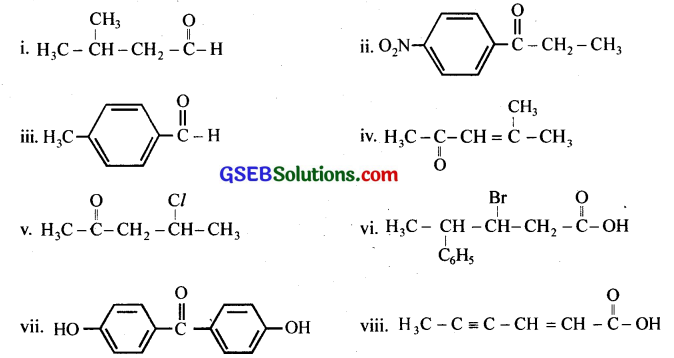
Question 2.
Draw the structures of the following compounds.
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) p, p’ -Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic aci
Answer:
Question 3.
Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CHBrCH2CH(CH3)CHO
(iii) CH3(CH3)5CHO
(iv) Ph-CH=CH-CHO
(v) 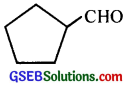
(vi) PhCOPh
Answer:
(i) Heptan-2-one
(ii) 4-Bromo-2-methylhexanal
(iii) Heptanal
(iv) 3-Phenylpropenal
(v) 7-Cyclopentanecarbaldehyde
(vi) Diphenylmethanone
![]()
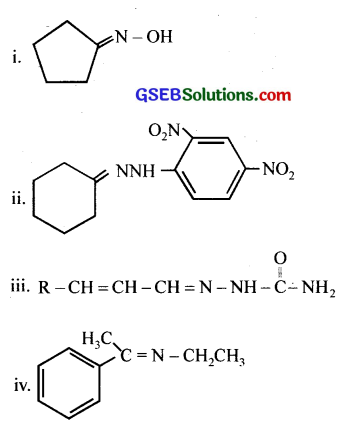
Question 4.
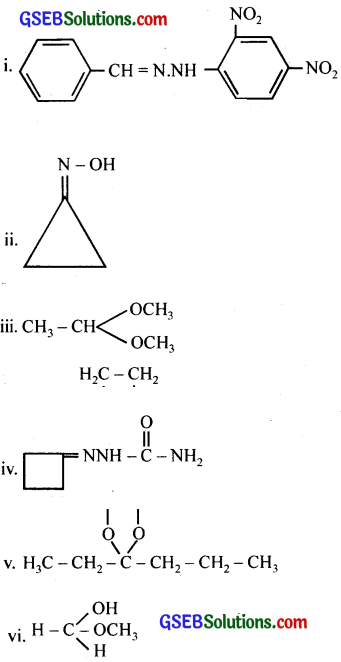
Draw structures of the following derivatives.
(i) The 2,4-dinitrophenylhydrazone ofbenzaldehyde
(ii) Cyclopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Answer:
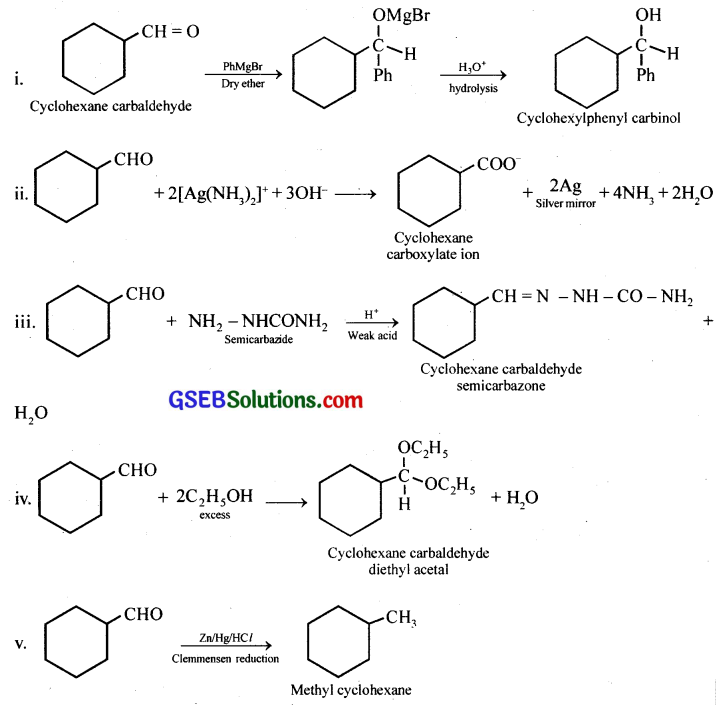
Question 5.
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii) Tollens’ reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Answer:
Question 6.
Which of the following compounds would undergo aldol condensation, with the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde
(viii) Butan-l-ol
(ix) 2,2-Dimethylbutanal
Answer:
(ii), (v), (vi), (vii): Aldol condensation, (i), (iii), (ix) Cannizaro reaction, (iv), (viii)Neither.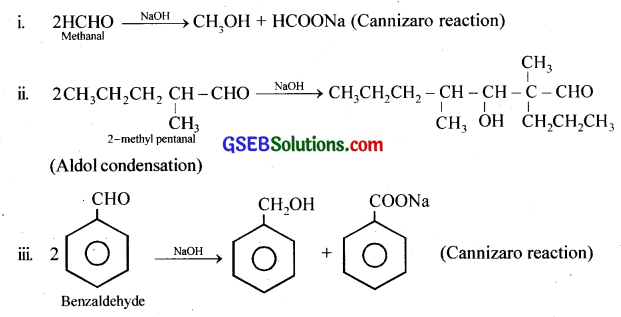
iv. Benzophenone will not undergo aldol condensation and Cannizaro reaction.
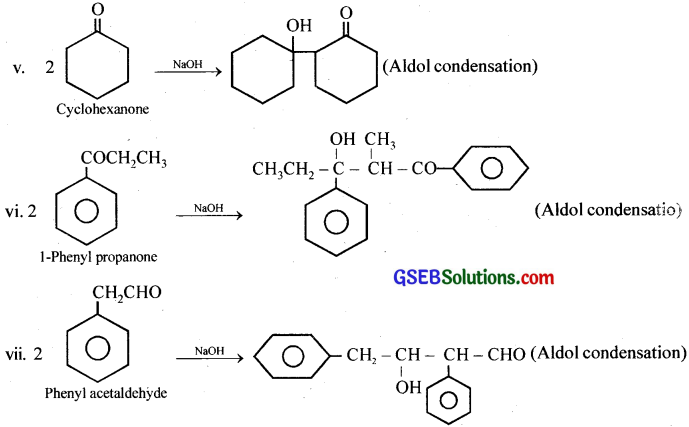
viii. Butan-l-ol will not undergo aldol condensation and Cannizaro reaction.
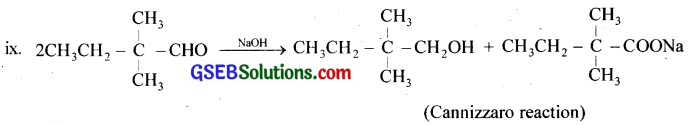
Question 7.
How will you convert ethanal into the following compounds?
(i) Butane-1,3-diol
(ii) But-2-enal
(iii) But-2-enoic acid
Answer:
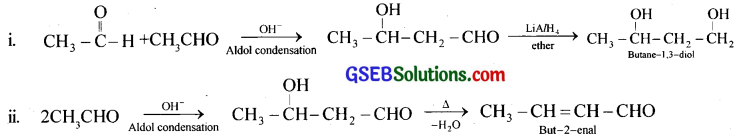
iii. But-2-enal obtained in (ii) is treated with chlorine in CCl4 in dark and the product obtained, is oxidised to dihalo acid which on dehydration gives but-2-enoic acid.
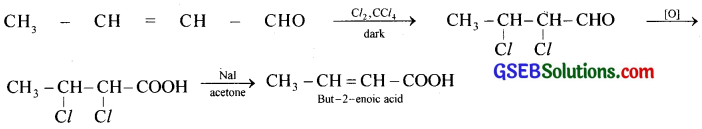
![]()
Question 8.
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
Answer:
‘A’ may be either butylbutanoate or (2-methyl propyl)-2-methyl propanoate
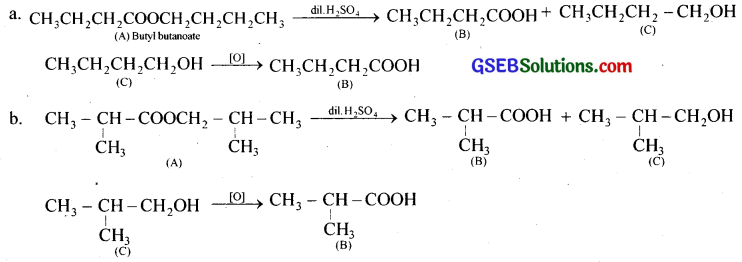
Question 9.
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
(i) Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde
(ii) (CH3)2CHCOOH < CH3CH2CH2COOH, < CH3CH(Br)CH2COOH
(iii) 4-Methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3,4-Dinitrobenzoic acid.
![]()
Question 10.
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Pentan-2-one and Pentan-3-one
(v) Benzaldehyde and Acetophenone
(vi) Ethanal and Propanal
Answer:
(i) Propanal and propanone – Propanal being an aldehyde reduces Tollen’s reagent to silver mirror but propanone being a ketone does not.
(ii) Acetophenone and benzophenone – Acetophenone gives yellow precipitate of iodoform but benzophenone does not.
![]()
(iii) Phenol and benzoic acid – Benzoic acid gives brisk effervescence with NaHC03 due to the formation of COz. Phenol does not.
C6H5COOH + NaHCO3 ? C6H5ONa + CO2 + H2O
(iv) Pentan-2-one and pentan-3-one – Pentan-2-one gives yellow precipitate of iodoform but pentan-3-one does not.
![]()
(v) Benzaldehyde and acetophenone – Acetophenone gives yellow precipitate of iodoform but benzaldehyde does not.
![]()
(vi) Ethanal and propanal – Ethanal forms yellow precipitate of iodoform while propanal does not.
![]()
Question 11.
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom,
(i) Methyl benzoate
(ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid
(iv) Phenylacetic acid
(v) p-Nitrobenzaldehyde.
Answer:
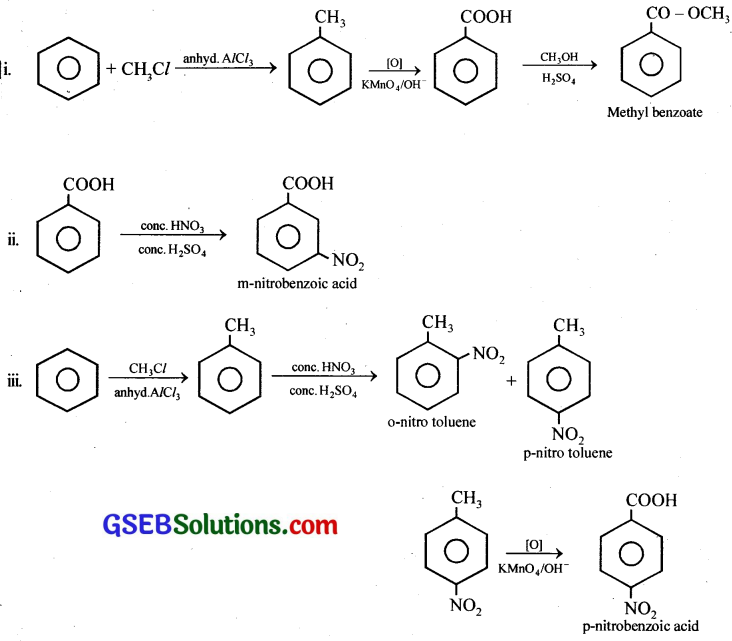
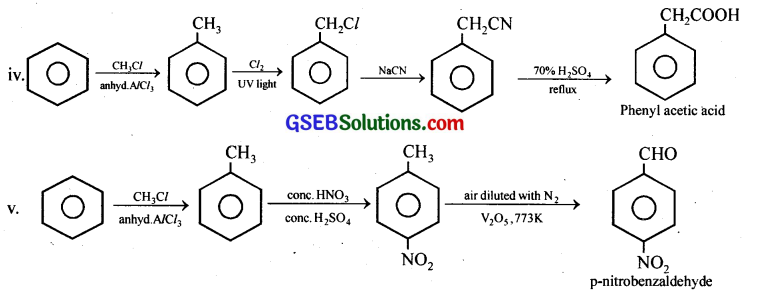
![]()
Question 12.
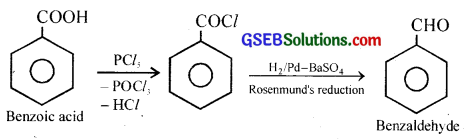
How will you bring about the following conversions in not more than two steps?
(i) Propanone to Propene
(ii) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(iv) Benzene to m-Nitroacetophenone
(v) Benzaldehyde to Benzophenone
(vi) Bromobenzene to 1 -Phenylethanol
(vii) Benzaldehyde to 3-Phenylpropan-l-ol
(viii) Benazaldehyde to a-Hydroxyphenylacetic acid
(ix) Benzoic acid to m-Nitrobenzyl alcohol
Answer:
(i) Propanone to propene:
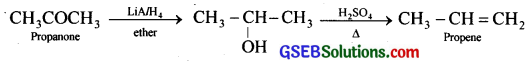
(ii) Benzoic acid to Benzaldehyde:
(iii) Ethanol to 3-Hydroxybutanal:

(iv) Benzene to m-Nitroacetophenone:
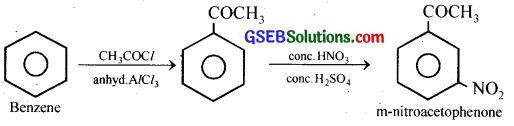
(v) Benzaldehyde to Benzophenone:
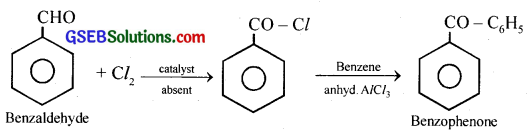
(vi) Bromobenzene to 1 -Phenylethanol:
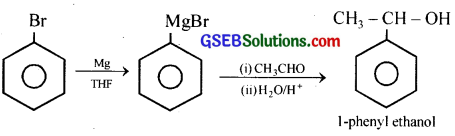
(vii) Benzaldehyde to 3-Phenylpropan-l-ol:
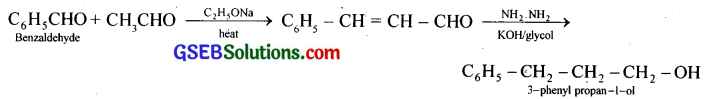
(viii) Benazaldehyde to a-Hydroxyphenylacetic acid:
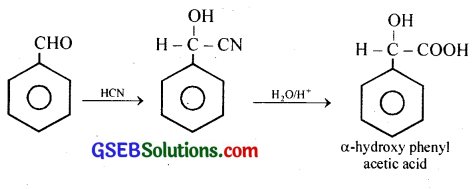
(ix) Benzoic acid to m-Nitrobenzyl alcohol:
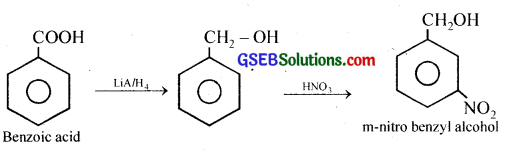
Question 13.
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
Answer:
(i) Acetylation – Fnedel Craft’s acylation reaction: Aromatic ketones are prepared by treating aromatic hydrocarbons with acid chlorides in the presence of anhydrous AlCl3.
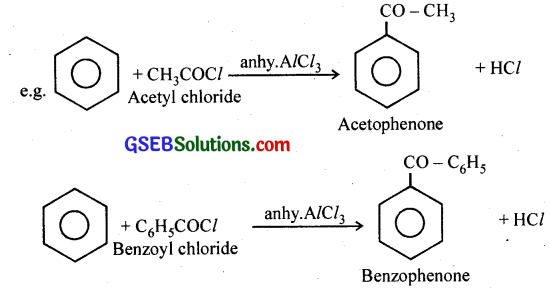
(ii) Cannizzaro reaction – Aldehydes which do not have any a -hydrogen atom (HCHO, C6H5CHO etc.) undergo self oxidation and reduction when treated with concentrated alkali to form the corresponding acid and alcohol. This reaction is called Cannizzaro reaction.
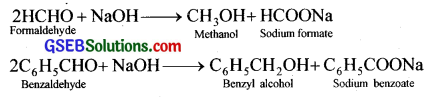
(iii) Cross aldol condensation – Crossed aldol condensation occurs (a) between two aldehydes or two ketones which are different, and (b) between an aldehyde and a ketone, provided they contain a – hydrogens. When a mixture of two different aldehydes is treated with base, four different aldol products are formed.
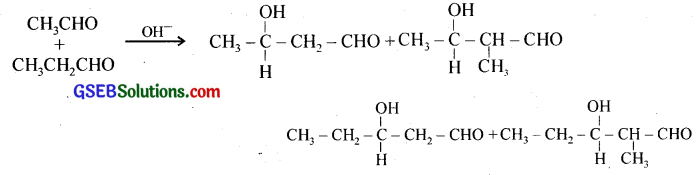
(iv) Decarboxylation – Decarboxylation is the elimination of CO2 from a carboxylic acid.
(a) When sodium salts of carboxylic acids are heated with soda lime (NaOH + CaO) alkanes are formed.
![]()
(b) When calcium salts of carboxylic acids are heated, aldehydes or ketones are formed.
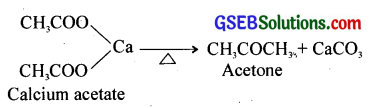
(c) Kolbe’s electrolysis – When aqueous solutions of sodium or potassium salts of carboxylic acids are electrolysed, higher alkanes are formed.
Example:
CH3COONa >CH3COO– + Na+
At anode: 2CH3COO– CH3CH3 + 2CO2 + 2e–
At cathode: 2Na+ + 2H2O + 2e– 2NaOH + H2
![]()
Question 14.
Complete each synthesis by giving missing starting material, reagent or products.
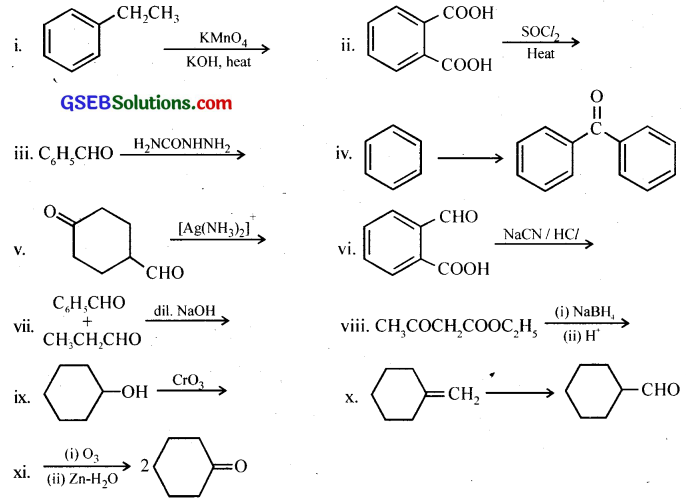
Answer:
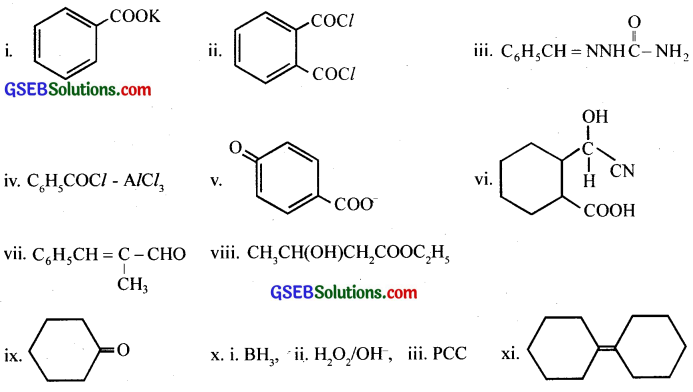
Question 15.
Give possible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not.
(ii) There are two – NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Answer:
(i) The presence of three electron donating methyl groups reduces electrophilicity of the carbonyl carbon to undergo nucleophilic addition. Also, the methyl groups hinder the attack of nucleophiles (sterric effect).
(ii) Among the two – NH2 groups present in semicarbazide, the nitrogen atom of the group distant from the carbon atom has high tendency to donate the lone pair electron. The nitrogen atom of the -NH2 group next to C = O group has weak tendency to donate the electron pair due to the partial positive charge on the adjacent carbon atom.
(iii) This is a reversible reaction and can be made to proceed to completion by removal of products (Le Chatelier’s principle).
![]()
Question 16.
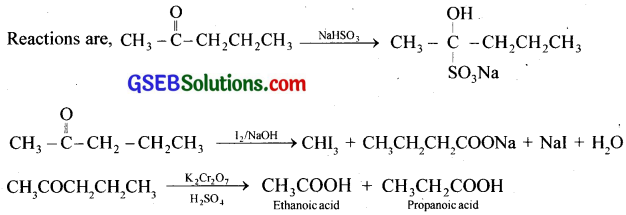
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Answer:
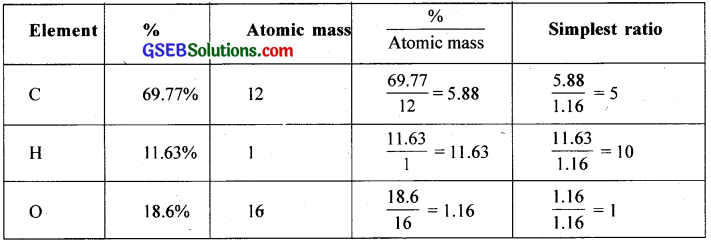
Empirical formula = C5H10O
Empirical formula mass = 86
Molecular mass (Given) = 86

= \(\frac { 86 }{ 86 }\) = 1
Molecular formula = C5H10O
(a) Since the given compound forms sodium hydrogen sulphite addition product, it must be either aldehyde or ketone.
(b) Since the compound does not reduce Tollen’s reagent, the compound cannot be an aldehyde and must be a ketone.
(c) Since the compound gives positive iodoform test, the given compound is a methyl ketone.
(d) Since the given compound on vigorous oxidation gives a mixture of ethanoic acid and propanoic acid, it is pentan-2-one.
CH3COCH2CH2CH3
Question 17.
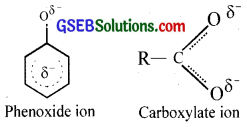
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Answer:
Acidity of carboxylic acids:
Carboxylic acids are acidic in nature. They can donate a proton and form salts with bases.

Carboxylic acids are weak acids. They are only partially ionised in aqueous solution and an equilibrium exists between the ionised and unionised forms.
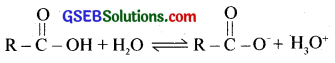
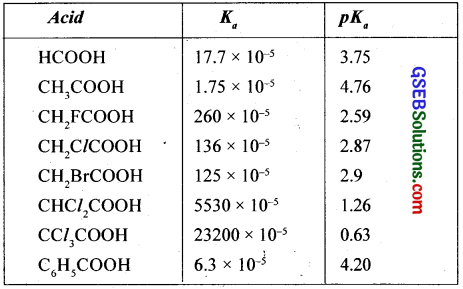
The extent of ionisation is described by an equilibrium constant Ka, which is known as acidity constant. It is defined as product of the concentrations of products of ionisation in moles – per litre divided by the concentration of the unionised acid.

The greater the value of Ka stronger will be the acid. The acid strength is more conveniently expressed as pKa = – log Ka.
As the pKa values increase, the strength of the acids decreases. The Ka and pKa values of some carboxylic acids are given below.
GSEB Class 12 Chemistry Aldehydes, Ketones and Carboxylic Acids Additional Important Questions and Answers
Question 1.
In two separate bottles, you are provided with ethanol and 2-propanol. Using these how will you prepare (i) ethanal (acetaldehyde) and (ii) acetone (propanone) by selecting suitable reagents?
Answer:
Ethanol on oxidation with acidified KMn04 or catalytic dehydrogenation using Cu tube/573K produces acetaldehyde.
![]()
2-propanol on oxidation with acidified KMn04 or catalytic dehydrogenation using Cu tube/573K produces acetone.
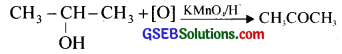
Question 2.
On ozonolysis of 1 mole of an alkene, a man got 2 moles of acetaldehyde. From this result, he proposed the following structure for the alkene.
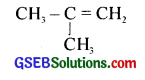
(i) Is it correct? Justify your answer.
(ii) Write the chemical equation.
(iii) If you get acetone and acetaldehyde as the products of ozonolysis, what would be the alkene?
Answer:
i. No.
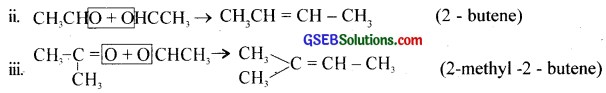
Question 3.
Discuss three reactions in which formaldehyde differs from other aldehydes.
Answer:
(a) Formaldehyde reacts with ammonia to give urotropine while other aldehydes give aldehyde ammonia.
![]()
(b) When treated with NaOH formaldehyde undergoes Cannizzaro reaction only while other aldehydes can undergo aldol condensation.
![]()
(c) Formaldehyde reacts with Grignard reagent to give l°alcohol while others give 2°alcohols.
![]()
Question 4.
In three test tubes, you are provided with formaldehyde, acetaldehyde, and acetone. How will you distinguish them?
Answer:
(i) Formaldehyde answers Tollen’s reagent test but does not answer the iodoform test.
(ii) Acetaldehyde answers both Tollen’s reagent test and iodoform test.
(iii) Acetone does not answer Tollen’s reagent test while answers the iodoform test.
![]()
Question 5.
(i) Why ketones are less reactive than aldehydes?
(ii) Benzaldehyde is less reactive than Acetaldehyde. Why?
Answer:
(i) Ketones are less reactive than aldehydes because in ketones there are two alky group attached with carbonyl group, due to the positive inductive effect (+ I) of both the alkyl group the positive charge on carbon atom decreases.
Hence, the sensitivity of ketones to the nucleophilic reagents decreases.
In aldehydes, they have only one alkyl group so they are more reactive than ketones.
(ii) —CHO group of benzaldehyde becomes stable due to resonance with benzene ring whereas resonance is not found in acetaldehyde. Benzaldehyde is aromatic and aldehyde is aliphatic.
Question 6.
Carboxylic acids are stronger than phenols. Why?
Answer:
The carboxylate ion formed from carboxilic acid is more resonance stabilised than the phenoxide ion formed from phenol. (In carboxylate ion, there are two electronegative oxygen atoms as compared to only one in phenoxide ion). Thus, the release of H+ ion from carboxylic acid is comparatively easier. Thus, carboxylic acids are stronger acid than phenols.
Question 7.
How will you convert
(i) acetic acid to ethyl alcohol
(ii) Carboxylate ion
(iii) acetaldehyde to isopropyl alcohol
(iv) acetone to tertiary butyl alcohol
(v) acetophenone to benzoic acid
Answer:
(i) When acetic acid is reduced with LiA/H4 in ether, ethanol is formed
![]()
(ii) When an amide is treated with bromine in presence of an alkali, a primary amine is formed.

(iii) When acetaldehyde is treated with methyl magnesium chloride an addition product is formed, which on hydrolysis gives isopropyl alcohol.
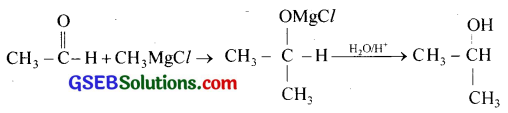
(iv) When acetone is treated with methyl magnesium chloride an addition product is formed, which on hydrolysis gives tertiary butyl alcohol.
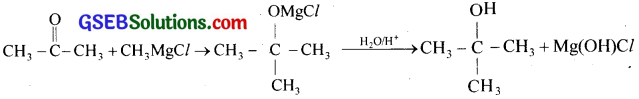
(v) When acetophenone is oxidised with alkaline KMnO4 or HNO3, benzoic acid is formed.
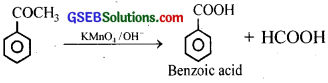
Question 8.
(i) Complete the table
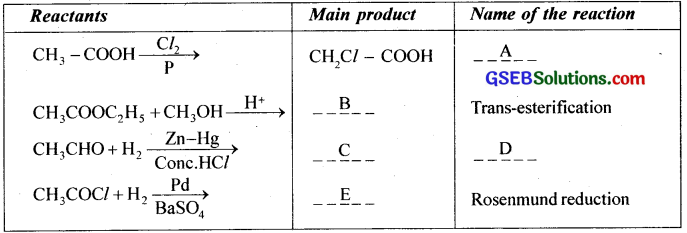
(ii) Illustrate Friedel Craft’s acylation reaction.
Answer:
(i) A – Hell – Volhard – Zelinsky (H-V-Z) reaction
B – CH3COOCH3
C – CH3 – CH3
D – Clemmensen reduction
E – CH3CHO
(ii) Aromatic hydrocarbons react with acid chloride in presence of anhydrous AlCl3 to form aromatic ketones. This reaction is called Friedel Craft’s acylation.

Question 9.
What is esterification?
How will you convert
(i) an ester to an amide
(ii) an ester to an acid chloride
Answer:
Carboxylic acids react with alcohols in the presence of a strong acid catalyst like H2SO4 to form esters. The reaction is reversible and the forward reaction is called esterification.
Example:
![]()
(i) An ester reacts with ammonia to give amide.
RCOOR’ + NH3 → RCONH2 + R’OH
(ii) An ester reacts with PC/5 to give an acid chloride.
RCOOR’ + PCl5 → RCOCl + R’OH + POCl3
![]()
Question 10.
How can you convert benzoic acid into
(i) 3-nitro benzoic acid
(ii) 3-sulpho benzoic acid
(iii) 3-Bromo benzoic acid?
Account for the above conversions.
Answer:
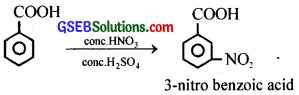
(i) When benzoic acid is treated with a mixture of conc. HNO3 and conc.H2SO4,
3-nitrobenzoic acid is formed.
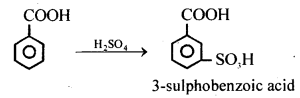
(ii) When benzoic acid is treated with cone. H2SO4, 3- sulphobenzoic acid is formed.
(iii) When benzoic acid is treated with bromine water, 3-bromobenzoic acid is formed.
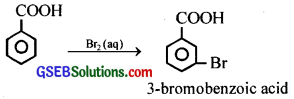
Since, -COOH is a meta directive group, the major product obtained in the above reaction is the meta derivative.
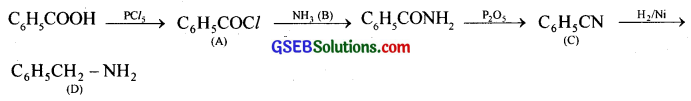
Question 11.
Identify A, B, C, and D from the following series of reactions.
![]()
Answer:
Question 12.
Identify A, B and C in the following reactions.
![]()
Answer:

Question 13.
A compound extracted from a plant has the molecular formula C3H6O2. It reacts with ammonia to form an amide and methanol.
(i) Identify the compound
(ii) Comment on the possibility of two methods of preparation of the compound from the compounds methanol, ethanoic acid and ethanoyl chloride.
(iii) Represent the chemical change when the compound is hydrolysed in presence of alkali.
Answer:
(i) The compound is CH3 – COOCH3 (methyl ethanoate)
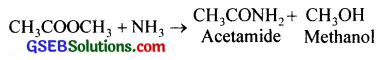
(ii)
(a) When methanol is treated with methanoic acid in presence of H2SO4 gives methyl ethanoate
![]()
(b) By the reaction between methanol and ethanoyl chloride gives methyl methanoate.
CH3COCl + CH3OH → CH3COOCH3 + HCl
(iii) When methyl ethanoate is treated with NaOH gives sodium acetate and methanol. This reaction is known as the saponification reaction.
CH3COOCH3 + NaOH → CH3COONa + CH3OH
![]()
Question 14.
Compare acidic strength of acetic acid, formic acid, and chloroacetic acid.
Answer:
The chlorine atom present in chloroacetic acid has a strong negative inductive effect (-I). Due to this, electrons of the O—H bond easily displaced towards oxygen and it releases H+ easily. CH3 group present in CH3COOH which produces (+ I) effect causes a decrease in acidic nature.
In formic acid there is no such group which produces (+ I) or (- I) effect. Hence, formic acid is stronger than acetic acid and chloroacetic acid is stronger than acetic acid. In short chloroacetic acid is stronger than formic acid and formic acid is stronger than acetic acid.
Question 15.
The percentage of C and H in an organic compound is in the ratio 6 : 1 and that of C and O is in the ratio 3 : 4.
(a) Identify the compound.
(b) What is the end product of the reaction between this compound and phenol? Explain the reaction.
(c) What are the uses of end products?
Answer:
(a) HCHO
(b) Phenol reacts with formaldehyde to give bakelite.

(c) It is a polymer used for making switches, combs etc.
![]()
Question 16.
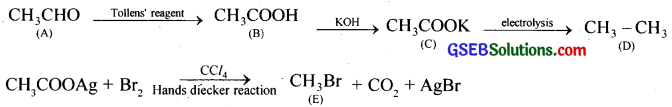
An organic compound having general formula C2 H4 O on oxidation with Tollens reagent produces B. B on treating with KOH produces C and C on electrolysis produces D. When silver salt of B is treated with Br2/CCl4 produces E. Identify A, B, C, D, E.
Answer: