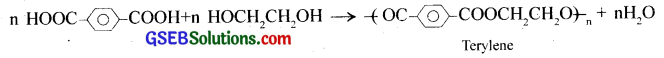Gujarat Board GSEB Textbook Solutions Class 12 Chemistry Chapter 15 Polymers Textbook Questions and Answers, Additional Important Questions, Notes Pdf.
Gujarat Board Textbook Solutions Class 12 Chemistry Chapter 15 Polymers
GSEB Class 12 Chemistry Polymers InText Questions and Answers
![]()
Question 1.
What are polymers?
Answer:
Polymers are high molecular mass substances (103-107u) formed by the combination of a large number of simple molecules. They are also called macromolecules. For example, polyethene, bakelite, PVC, Teflon, etc.
Question 2.
How are polymers classified on the basis of structure?
Answer:
On the basis of structure, the polymers are classified as
- Linear polymers such as polythene, polyvinyl chloride, etc.
- Branched-chain polymers such as low-density polythene
- Cross-linked polymers such as bakelite, melamine, etc.
![]()
Question 3.
Write the names of monomers of the following polymers:
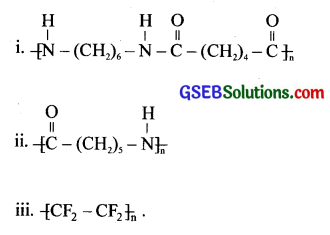
Answer:
(i) Hexamethylene diamine and adipic acid.
(ii) Caprolactam
(iii) Tetrafluoroethene
Question 4.
Classify the following as addition and condensation polymers: Terylene, Bakelite, Polyvinyl chloride, Polythene.
Answer:
- Terylene: Condensation polymer
- Bakelite: Condensation polymer
- Polyvinyl chloride: Addition polymer
- Polyethylene: Addition polymer.
Question 5.
Explain the difference between Buna – N, and Buna – S.
Answer:
Buna – N is a copolymer of 1, 3 – butadiene and acrylonitrile and Buna – S is a copolymer of 1, 3 – butadiene and styrene.
Question 6.
Arrange the following polymers in increasing order of their intermolecular forces.
- Nylon – 66, Buna-S, Polythene.
- Nylon – 6, Neoprene, Polyvinylchloride.
Answer:
- Buna-S < Polyethene < Nylon-6,6.
- Neoprene < Polyvinyl chloride < Nylon-6.
![]()
Question 7.
Is [CH2 – CH(C6H5) ]n a homopolymer or a copolymer?
Answer:
It is a homopolymer and the monomer from which it is obtained is styrene C6H5CH = CH2.
GSEB Class 12 Chemistry Polymers Text Book Questions and Answers
Question 1.
Explain the terms polymer and monomer.
Answer:
(i) Polymer is a high molecular mass macromolecule consisting of repeating structural units derived from monomers.
(ii) Monomer is a simple molecule capable of undergoing polymerisation and leading to the formation of the corresponding polymer.
Question 2.
What are natural and synthetic polymers? Give two examples of each type.
Answer:
(i) Natural polymers are high molecular mass macromolecules and are found in plants and animals. Examples are proteins and nucleic acids.
(ii) Synthetic polymers are man-made high molecular mass macromolecules. These include synthetic plastics, fibres and rubbers. The two specific examples are polythene and dacron.
![]()
Question 3.
Distinguish between the terms homopolymer and copolymer and give an example of each. (CBSE 2008)
Answer:
(i) A polymer which is obtained from only one type of monomer molecules is known as homopolymer. example Polyethene
![]()
(ii) A polymer which is obtained from two or more types of monomer molecules is known as copolymer, example Buna – S
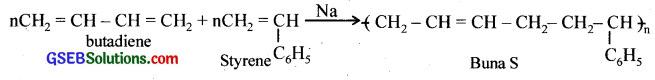
Question 4.
How do you explain the functionality of a monomer?
Answer:
Functionality is the number of bonding sites in a monomer.
Question 5.
Define the term polymerisation. (CBSE 2008)
Answer:
Polymerisation is a process of formation of a high molecular mass polymer from one or more monomers by linking together of repeating structural units with covalent bonds.
Question 6.
Is ![]() , a homopolymer or copolymer?
, a homopolymer or copolymer?
Answer:
Since the unit ![]() is obtained from a single monomer unit, it is a homopolymer.
is obtained from a single monomer unit, it is a homopolymer.
![]()
Question 7.
In which classes, the polymers are classified on the basis of molecular forces?
Answer:
On the basis of molecular forces present between the chains of various polymers, the classification of poly mers is given as follows.
- Elastomers
- Fibres
- Thermoplastics
- Thermosetting plastics
Question 8.
How can you differentiate between addition and condensation polymerisation?
Answer:
In addition polymerisation, the molecules of the same or different monomers add together to form a large polymer molecule. Condensation polymerisation is a process in which two or more bi – functional molecules undergo a series of condensation reactions with the elimination of some simple molcules and leading to the formation of polymers.
Question 9.
Explain the term copolymerisation and give two examples.
Answer:
Co – polymerisation is a process in which a mixture of more than one monomeric species is allowed to polymerise. The copolymer contains multiple units of each monomer in the chain. The examples are copolymers of 1, 3 – butadiene and styrene and 1, 3 – butadiene and acrylonitrile.
![]()
Question 10.
Write the free radical mechanism for the polymerisation of ethene.
Answer:
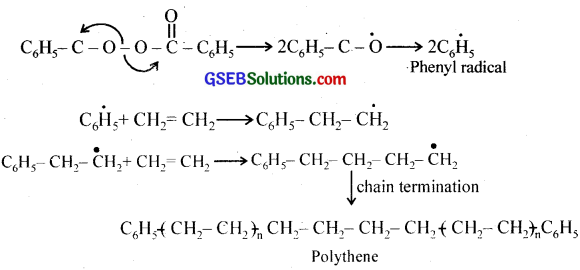
Question 11.
Define thermoplastics and thermosetting polymers with two examples of each. (CBSE 2008)
Answer:
(i) A thermoplastic polymer can be repeatedly softened on heating and hardened on cooling, hence it can be used again and again. The examples are polythene, polypropylene, etc.
(ii) A thermosetting polymer is a permanent setting polymer as it gets hardened and sets during moulding process and cannot be softened again. The examples are bakelite and melamine formaldehyde polymers.
Question 12.
Write the monomers used for getting the following polymers,
(i) Polyvinyl chloride
(ii) Teflon
(iii) Bakelite
Answer:
(i) The monomer of polyvinyl chloride is CH2 = CHCl (vinyl chloride)
(ii) The monomer of teflon is CF2 = CF2 (tetrafluoroethylene)
(iii) The monomers involved in the formation of bakelite are HCHO (formaldehyde) and C6H5OH (phenol)
Question 13.
Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Answer:
Benzoyl peroxide is a common initiator used in free radical addition polymerisation.

Question 14.
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Answer:
From the structural point of view, natural rubber is a linear cis – 1, 4 – polyisoprene. In this polymer the double bonds are located between C2 and C3 of isoprene units. This cis – configuration about double bonds do not allow the chains to come closer for effective attraction due to weak intermolecular attractions. Hence, the natural rubber has a coiled structure and shows elasticity.
![]()
Question 15.
Discuss the main purpose of vulcanisation of rubber.
Answer:
Vulcanisation is done to improve the elasticity, reduce water-absorption tendency and increase the resistance to oxidation and organic solvents to rubber. During vulcanisation, sulphur forms cross links between the polymer chains thereby making the rubber more stiff.
Question 16.
What are the monomeric repeating units of Nylon – 6 and Nylon – 6,6?
Answer:
The monomeric repeating unit of Nylon-6 polymer is: [NH – (CH2)5 – CO] The monomeric repeating unit of Nylon-6,6 polymer is derived from the two monomers, hexamethylene diamine and adipic acid.
![]()
Question 17.
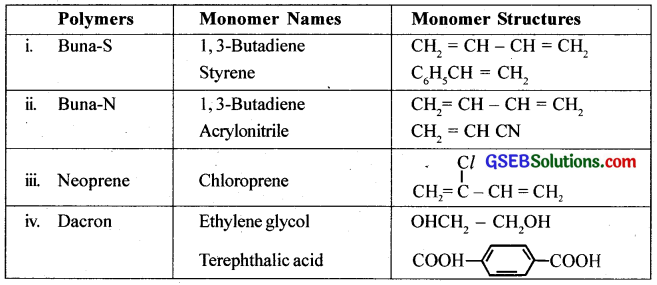
Write the names and structures of the monomers of the following polymers: (CBSE 2008)
(i) Buna – S
(ii) Buna – N
(iii) Dacron
(iv) Neoprene
Answer:
The names and structures of monomers are:
Question 18.
Identify the monomer in the following polymeric structures.
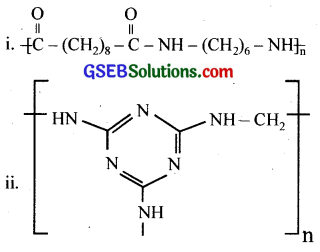
Answer:
The monomers forming the polymer are:
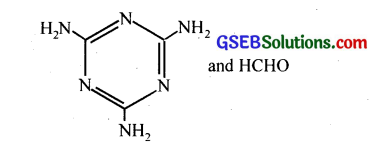
(i) Decanoic acid (HOOC – (CH2)8 – COOH) and Hexamethylene diamine (H2N(CH2)6NH2)
(ii)
Question 19.
How is dacron obtained from ethylene glycol and terephthalic acid?
Answer:
Dacron or terylene is obtained by condensation polymerisation of ethylene glycol and terephthalic acid. The reaction is carried out at 420 – 460K in presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide.
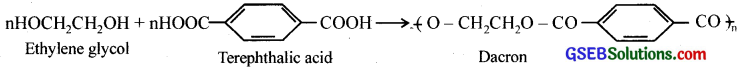
Question 20.
What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Answer:
A polymer which degrades in the biological systems by enzymatic hydrolysis or oxidation is called a biodegradable polymer. example PHBV – polyhydroxybutyrate – co – p – hydroxyvalerate
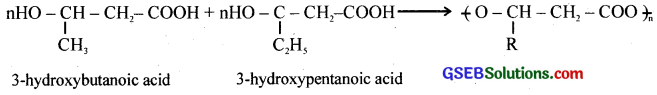
where R = – CH3, or – C2H5
GSEB Class 12 Chemistry Polymers Additional Important Questions and Answers
Question 1.
Natural rubber is an elastomer.
(i) Briefly explain the properties of elastomers.
(ii) Write the monomer present in natural rubber.
(iii) If you are asked to make natural rubber hard, how will you make it hard? Explain the chemistry behind it.
Answer:
(i) Polymers having elasticity like rubber are termed elastomers. In elastomers the polymeric chains are held together by weak intermolecular forces. Elastomers are characterised by their ability to be elongated under stress and to regain its original shape when the stress is removed.
(ii) Isoprene (2 – methyl – 1, 3 – butadiene)
(iii) Natural rubber can be made hard by vulcanisation i.e, by heating it with sulphur. When natural rubber is heated with sulphur, S – cross links are formed between the chains. Cross linked polymers are usually rigid and hard.
![]()
Question 2.
In a factory the supervisor told the technical assistant that they got a bulk order for polythene.
Then the technical assistant asked whether it was LDPE or HDPE.
(i) What do the abbreviations stand for?
(ii) How are they prepared? Give equation?
(iii) List out their applications.
Answer:
(i) Low-density polyethylene (LDPE) and high-density polyethylene (HDPE).
(ii) a. LDPE
When ethylene undergoes polymerisation in presence of oxygen at high pressure and high-temperature LDPE is formed. LDPE is a branched chain polymer.

b. HDPE. When ethylene is heated under high pressure in the presence of a catalyst, it polymerizes to polyethylene. It is HDPE. Catalyst used is the Ziegler-Natta catalyst.
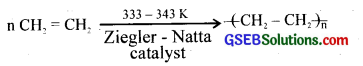
(iii) LDPE is flexible and is used as translucent films for packing and in the manufacture of pipes, bottles etc. High-density polythene is hard, tough and chemically inert. It is used in making toys, pipes and household articles.
Question 3.
How does thermoplastic polymer differ from thermosetting polymer?
Answer:
Difference between Thermoplastic polymer and Thermosetting polymer:
Thermoplastic:
- These are linear polymers.
- Weak van der Waals’ force is present in the form of intermolecular force which on heating becomes soft and melts.
- It can be remoulded into different shapes again and again. Example: Polystyrene, P.V.C., terylene etc.
Thermosetting:
- These are cross-linked polymers.
- Due to the presence of chemically cross-linked bonds, thermosetting polymers become non-fusible and do not melt on heating.
- They become hard at the time of moulding and therefore cannot be remoulded. Example: Formaldehyde, resin, glyptal etc.
Question 4.
Identify me.
- I am a polymer resistant to heat and chemicals. People use me to make non-sticky frying pans.
- I am a polymer formed from ethylene glycol and terephthalic acid, used for making heart valves.
- I am a polymer used for making unbreakable crockery items.
Answer:
- Teflon
- Dacron
- Melamine formaldehyde resin
Question 5.
List some important differences between Natural rubber and the Vulcanization of rubber.
Answer:
Differences between Natural rubber and Vulcanisation of rubber:
Natural rubber:
- It is soft and sticky.
- It has low tensile strength.
- It has low elasticity.
- It can be used over a narrow range of temperatures (10°C to60°C).
Vulcanisation of rubber:
- It is hard and non-sticky.
- It has high tensile strength.
- It has high elasticity.
- It can be used over a wide range of temperatures (40°C to 100°C).
![]()
Question 6.
Write a note on
(i) polyamides
(ii) polyesters
Answer:
(i) Polyamides – Polymers having amide linkages -(CO-NH )- in their chain are called polyamides, for example nylon- 66, nylon-6, etc. Nylon-66 is a polyamide formed between hexa-methylene diammine and adipic acid.
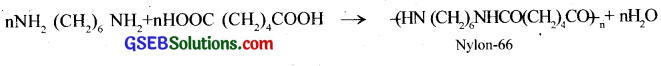
(ii) Polyesters – Polymers having ester linkages -(CO-O)- in their chain are called polyesters, for example terylene, glyptal, etc. Terylene is a polyester of ethylene glycol and terephthalic acid.