Gujarat Board GSEB Textbook Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure Textbook Questions and Answers, Notes Pdf.
Gujarat Board Textbook Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure
Gujarat Board Class 9 Science Is Matter Around Us Pure InText Questions and Answers
Page – 15
Question 1.
What is meant by a pure substance?
Answer:
A pure substance is the one that consists of a single type of particle, i.e., all constituent particles of the substance have the same chemical nature. Pure substances can be classified as elements or compounds.
![]()
Question 2.
List the points of difference between homogeneous and heterogeneous mixtures.
Answer:
Homogeneous mixtures:
- Homogeneous mixtures have uniform composition.
- It has no visible boundaries of separation between its constituents.
Heterogeneous mixtures:
- Heterogeneous mixtures have non-uniform composition.
- It has visible boundaries of separation between its constituents.
Page – 18
Question 3.
Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
A homogeneous mixture is a mixture having a uniform composition throughout the mixture. For example, mixtures of salt in water, sugar in water, copper sulphate in water, iodine in alcohol, alloy and air have uniform composition throughout the mixtures.
On the other hand, a heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture. For example, composition of mixtures of sodium chloride and iron filings, salt and sulphur, oil and water, chalk powder in water, wheat flour in water, milk and water are not uniform throughout the mixtures.
![]()
Question 4.
How are sol, solution and suspension different from each other?
Answer:
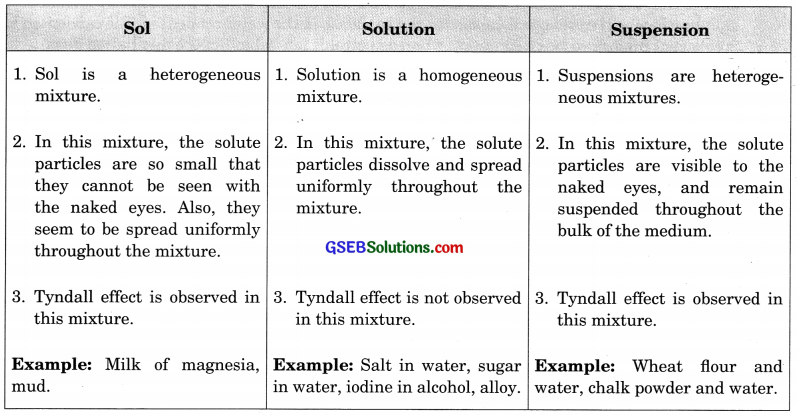
Question 3.
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Concentration means amount of a solute dissolved in 100 g of the solvent. Here, 36 g of sodium chloride is dissolved in 100 g of water, therefore, concentration of the solution is 36 g per 100 g of water.
Page – 24
Question 4.
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer:
Kerosene and petrol and miscible liquids also the difference between their boiling point is more than 25°C so they can be separated by the method of distillation.
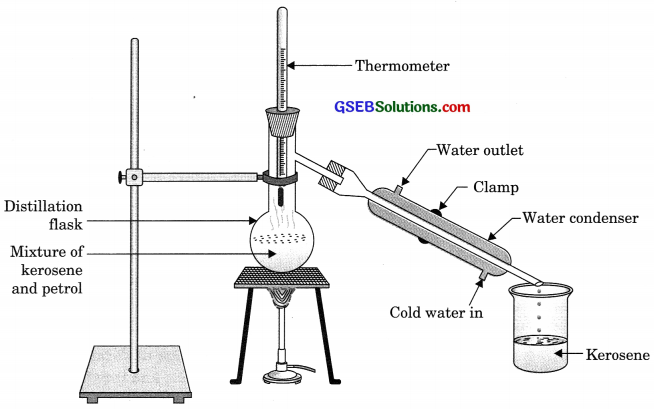
In this method, the mixture of kerosene and petrol is taken in a distillation flask with a thermometer fitted in it. We also need a beaker, a water condenser, and a Bunsen burner. The apparatus is arranged ‘ as shown in the above figure. Then, the mixture is heated slowly. The thermometer should be watched simultaneously. Kerosene will vapourise and condense in the water condenser. The condensed kerosene is collected from the condenser outlet, whereas petrol is left behind in the distillation flask.
Question 5.
Name the technique to separate
(i) butter from curd
(ii) salt from sea water
(iii) camphor from salt.
Answer:
(i) Butter can be separated from curd by centrifugation.
(ii) Salt can be separated from sea water by evaporation.
(iii) Camphor can be separated from salt by sublimation.
Question 6.
What type of mixtures are separated by the technique of crystallisation?
Answer:
Solids, in which the impurities are either insoluble or are more soluble than the solids in a particular solvent, can be easily separated by the technique of crystallisation. For example, impure copper sulphate, alum, nitre, sea salt, etc., can be easily purified by the technique of crystallisation.
![]()
Page – 24
Question 7.
Classify the following as chemical or physical changes:
(i) cutting of trees,
(ii) melting of butter in a pan,
(iii) rusting of almirah,
(iv) boiling of water to form steam,
(v) passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
(vi) dissolving common salt in water,
(vii) making a fruit salad with raw fruits, and
(viii) burning of paper and wood.
Answer:
(i) Physical change
(ii) Physical change
(iii) Chemical change
(iv) Physical change
(v) Chemical change
(vi) Physical change
(vii) Physical change
(viii) Chemical change.
Question 8.
Try segregating the things around you as pure substances or mixtures.
(a) distilled water
(b) curd
(c) diamond
(d) ice-cream
(e) kerosene oil
(f) cooking oil
(g) steel
(h) graphite
(i) raw rubber
(j) vulcanized rubber
(k) solder wire.
Answer:
Pure substances – Distilled water, diamond, graphite, raw rubber.
Mixtures – Curd, ice-cream, kerosene oil, cooking oil, steel, vulcanized rubber, solder wire (alloy of lead and tin).
![]()
In-Text Activities Solved
(Textbook Page 1)
Activity 2.1
Answer:
Observation: Group A and B have obtained a mixture which has a uniform composition throughout. Such mixtures are called homogeneous mixtures or solutions. Though both the groups, i.e., A and B have obtained copper sulphate solution but the intensity of colour of the solutions is different. Different intensity of colour of the solutions shows that a homogeneous mixture can have a variable composition. Groups C and D have obtained mixtures, which contain physically distinct parts and have non-uniform compositions. Such mixtures are called heterogeneous mixtures.
Conclusion: A mixture which has a uniform composition throughout. Such mixtures are called homogeneous mixtures or solutions.
A mixture which has variable composition throughout. Such mixtures are called heterogeneous mixtures or solutions.
(Textbook Page 15)
Activity 2.2
Answer:
Observation:
- The particles in case of group A and B will not be visible whereas in group C and D it would be visible.
- In case of group A and B, the particles will not show path of the beam of light whereas in group C and D the particles will show the path of beam of light from a torch.
- The mixture is stable only in case of group A and B, as it does not settle down when kept for some time.
- For D it is not observed rapidly. On filtering the mixture, residue is left on the filter paper in case of group C.
Conclusion: Groups A and B have true solutions. Group C has suspension. Group D has got a colloidal solution.
(Textbook Page 16)
Activity 2.3
Answer:
Observation: When no more solute can be dissolved in a solution at a given temperature, it is called a saturated solution. So, at any particular temperature, a solution that has dissolved as much solute as it is capable of dissolving is said to be a saturated solution.
No, the amount of salt and sugar or barium chloride that can be dissolved in water at a given temperature is not the same. By increasing the temperature more solute can be added.
Conclusion: The amount of the solute present in the saturated solution at the given temperature is called its solubility. We can infer from the activity that different substances in a given solvent have different solubilities at particular temperature.
(Textbook Page 19)
Activity 2.4
Answer:
Observation: The liquid present in the watch glass evaporates on heating. A residue of ink will be left on the watch glass in a dry state. We can separate the volatile component (solvent) from its non-volatile solute by the method of evaporation. Ink is a mixture of a dye in water or a solvent.
Conclusion: From this activity we can conclude that ink is not a single substance, but is a mixture of dye and water/ solvent.
(Textbook Page 19)
Activity 2.5
Answer:
Observation: The number of soluble fats present in the milk collides with one smother at high speed to form bigger particles in the form of cream. The cream formed get separated and leaves behind the fat free milk. Centrifugation works on the principle that the denser particles are forced to move to the bottom and the lighter particles stays at the top of the test tube when spin rapidly.
Conclusion: Cream can be separated from milk by the process of centrifugation.
![]()
(Textbook Page 20)
Activity 2.6
Answer:
Observation: Two distinct layers of kerosene oil and water are formed when separating funnel is left undisturbed for some time. This process is based on the principle that immiscible liquids separate out in layers depending on their densities. Heavier density liquid remains in bottom as lower layer while the lighter density liquid present as upper layer.
Conclusion: A separating funnel helps in separating two immiscible liquids based on the difference in the densities of the liquids in the mixture.
(Textbook Page 21)
Activity 2.7
Answer:
Observation: The ink that we use has water as the solvent and the dye is soluble in it and so when the water rises on the filter paper it takes along with it the dye particles. Yes, different coloured spots of dyes are obtained because a dye is a mixture of two or more colours. The coloured component is more soluble in water; therefore it rises faster on the paper strip.
Conclusion: Coloured components of a mixture can be separated by using chromatography.
(Textbook Page 21)
Activity 2.8
Answer:
Observation: The thermometer shows an increase in the temperature. At 56.5°C, the thermometer reading becomes constant for some time. At this temperature, acetone vapourises, condenses and can be collected from the condenser outlet. Water is left behind in the distillation flask. Acetone boils at 56.5°C while water boils at 100°C. The two components of the mixture separate because acetone is more volatile than water.
Conclusion: The distillation process is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
(Textbook Page 23)
Activity 2.9
Answer:
Observation: The crystals of pure copper sulpahte in the china dish are observed. The crystals are not alike. The pure crystals can be separated from the liquid in the china dish by filtration.
Conclusion: Pure crystals of copper sulpahte can be obtained from impure sample by the process of crystallisation.
(Textbook Page 25)
Activity 2.10
Answer:
Observation: The material obtained by group 1 is a mixture of the two substances, i.e., iron and sulphur. The material obtained by group 2 is a compound. The properties of the mixture are the same as that of its constituents. We can also observe that the texture and the colour of the compound is the same throughout. A black substance is formed and it does not get attracted to the magnet any more. Hence, group 1 obtained a material with magnetic properties.
The gas obtained by group 1 is hydrogen gas. It is colourless, odourless and combustible gas. So, it is not advised to do the combustion test for hydrogen in the class. The gas obtained by group 2 is hydrogen sulphide. It is a colourless gas with the smell of rotten eggs. Though the starting materials were same but the product obtained by both the groups showed different properties. The group 1 has carried out the activity involving a physical change whereas in group 2, a chemical change has taken place.
Conclusion: Heat is not needed for the formation of mixture but it is needed for the formation of the compound.
Gujarat Board Class 9 Science Is Matter Around Us Pure Textbook Questions and Answers
Question 1.
Which separation techniques will you apply for the separation of the following?
(а) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
(a) Crystallisation or Evaporation
(b) Sublimation
(c) Filtration
(d) Chromatography
(e) Centrifugation
(f) Separating funnel
(g) Filtration
(h) Magnetic separation
(i) Blowing air
(j) Using alum.
![]()
Question 2.
Write the steps you would use for making tea. Use the words-solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
Take the solvent, water, in a kettle, heat it. When the solvent boils, add the solute, milk. Milk and water form a solution. Then pour some tea leaves over a sieve. Slowly pour the hot solution of milk over tea leaves. Colour of tea leaves goes into solution as filtrate. The remaining tea leaves being insoluble remains as residue. Add requisite sugar which dissolves and the tea is ready.
Question 3.
Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (result are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
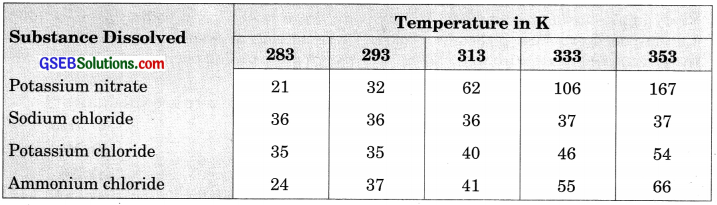
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
(a) At 313K,
Potassium nitrate needed to produce a saturated solution in 100 grams of water = 62 g
∴ Amount of potassium nitrate to produce a saturated solution in 50 grams of water = 31 g
(b) Some amount of dissolved potassium chloride will reappear as undissolved solid as solubility of solute decreases with the decrease of temperature.
(c) Solubility of each salt at 393 K is as follows:
- Potassium nitrate – 32
- Sodium chloride – 36
- Potassium chloride – 35
- Ammonium chloride – 37
Ammonium chloride salt has the highest solubility at this temperature.
(d) Solubility of a salt increases with the increase in temperature.
Question 4.
Explain the following giving examples.
(a) saturated solution
(b) pure substance
(c) colloid
(d) suspension
Answer:
(а) A solution in which no more solute can be dissolved at a particular temperature is known as a saturated solution. For example, in aqueous solution of sugar no more sugar can be dissolved at room temperature.
(b) A pure substance is a substance consisting of a single type of particles, i.e., all constituent particles of the substance have the same chemical properties. For example, water, sugar, salt, etc.
(c) A colloid is a heterogeneous mixture whose particles are not as small as solution but they are so small that they cannot be seen, with the naked eyes. When a beam of light is passed through a colloid then the path of the light becomes visible. For example, milk, smoke, etc.
(d) A suspension is a heterogeneous mixture in which solids are dispersed in liquids. The solute particles in suspension do not dissolve but remain suspended throughout the medium. For example, paints, muddy water, chalk water mixtures, etc.
![]()
Question 5.
Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air, soil, vinegar, filtered tea.
Answer:
(a) Homogeneous mixtures → Soda water, vinegar and filtered tea are homogeneous mixtures. Air is also a homogeneous mixture if dust particles and other suspended impurities are excluded.
(b) Heterogeneous mixtures → Wood and soil are heterogeneous mixtures. Air is also a heterogeneous mixture if dust particles and other suspended impurities are included.
Question 6.
How would you confirm that a colourless liquid given to you is pure water?
Answer:
Every liquid has a characteristic boiling point. Pure water has a boiling point of 100°C (373 K) at 1 atmospheric pressure. If the given colourless liquid boils at even slightly above or below 100°C, then the given liquid is not pure water. Thus, by observing the boiling point, we can confirm whether a given colourless liquid is pure water or not.
Question 7.
Which of the following materials falls in the category of a “pure substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Answer:
The following materials fall in the category of a “pure substance”.
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
Question 8.
Identify the solutions among the following mixtures:
(a) Soil
(b) Seawater
(c) Air
(d) Coal
(e) Soda water.
Answer:
The following mixtures are solutions:
(b) Seawater
(c) Air
(e) Soda water.
Question 9.
Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution.
Answer:
Tyndall effect is shown by colloidal solutions. Here milk and starch solution are colloids, therefore they will show Tyndall effect.
Question 10.
Classify the following into elements, compounds and mixtures:
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood.
Answer:
Elements: Sodium, silver, tin and silicon.
Compounds: Calcium carbonate, methane and carbon dioxide.
Mixtures: Soil, sugar solution, coal, air, soap and blood.
![]()
Question 11.
Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Answer:
The following changes are chemical changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion food
(g) Burning of a candle.