Gujarat Board GSEB Textbook Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations Textbook Questions and Answers, Additional Important Questions, Notes Pdf.
Gujarat Board Textbook Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations
Gujarat Board Class 10 Science Chemical Reactions and Equations InText Questions and Answers
Question 1.
Why should a magnesium ribbon be cleaned before burning in air?
Answer:
A magnesium ribbon should be cleaned before burning in air because magnesium reacts with oxygen slowly to form magnesium oxide which prevents the burning of magnesium. The layer of magnesium oxide should be removed by sand paper.
Question 2.
Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride.
Answer:
H2 + Cl2; 3BaCl2 + Al2 (SO4)3 → 3BaSO4 + 2AlCl3
![]()
Question 3.
Write a balanced chemical equation with state symbols for the following reaction.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer:
(i) BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
(ii) NaOH(aq) + HCl(1) → NaCl(aq) + H2O(l)
Question 4.
A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Answer:
(i) X is quick lime (CaO) which is used for white washing.
(ii) CaO + H2O → Ca(OH)2
Question 5.
Why is the amount of gas collected in one of the test tube in Activity 1.7 double of the amount collected in the other? Name the gas.
Answer:
It is because water is formed when hydrogen and oxygen combine in the ratio of 2:1 by volume, the gas with double volume is hydrogen. Thus, on decomposition of water the volume of hydrogen formed is twice than that of oxygen gas.
Question 6.
Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer:
Copper sulphate solution is blue in colour when an iron nail is dipped in it, its blue colour changes. This happens due to the displacement reaction where iron being more reactive than Cu, displaces it to form green coloured iron sulphate solution and copper metal.
![]()
![]()
Question 7.
Give an example of a double displacement reaction other than the one given in Activity
Answer:
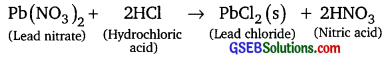
Question 8.
Identify the substance that are oxidised and the substances that are reduced in the following reactions.
(i) 4Na(s) + O2(g) → 2Na2O(s)
(ii) CuO(s) + H2(g) → Gu(s) + H2O(1)
Answer:
(i) Sodium metal is oxidised and oxygen is reduced to Na2O.
(ii) Copper oxide is reduced to copper and hydrogen is oxidised to H2O.
![]()
In-Text Activities Solved
Activity 1.1
Answer:
Magnesium ribbon burns with dazzling light and substance formed is magnesium oxide.
2Mg(s) + O2(g) → 2MgO(s)
Activity 1.2
Answer:
Lead nitrate reacts with potassium iodide to form lead iodide which is insoluble in water and yellow in colour.
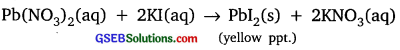
Activity 1.3
Answer:
When zinc metal reacts with dilute hydrochloric acid then zinc chloride is formed and bubbles of hydrogen gas are observed. The conical flask becomes hot which shows that the reaction is exothermic.
![]()
Activity 1.4
Answer:
Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing a large amount of heat.
CaO(s) + H2O(1) → Ca(OH)2(aq) + ∆
The reaction is combination in nature as calcium oxide reacts with water to form a single product, calcium hydroxide.
![]()
Activity 1.5
Answer:
The green colour of ferrous sulphate crystals changes to brownish-black ferric oxide and smell of burning sulphur dioxide is observed
![]()
Activity 1.6
Answer:
Pungent smelling, brown fumes are evolved due to N02 gas and brownish residue of lead oxide (PbO) is left.
2Pb(NO3)2(s) 2PbO(s) + 4NO2(g) + O2(g)
Activity 1.7
Answer:
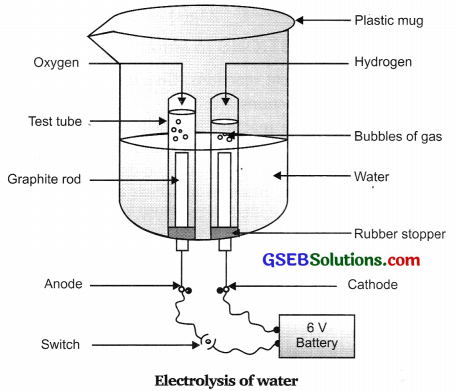
The volume of one of the gases i.e., hydrogen, is twice the volume of the other gas, oxygen. One of the gases catches fire and burns with pop sound (hydrogen) whereas in other gas, candle bums brightly (oxygen). The gas collected at anode is oxygen and the gas collected at cathode is hydrogen.
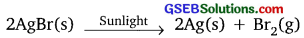
Activity 1.8
Answer:
White silver chloride turns grey in sunlight because silver metal is formed due to photolytic reaction and chlorine gas is evolved.
![]()
Activity 1.9
Answer:
Copper sulphate solution is blue, when an iron nail is dipped in it, its blue colour changes. This happens due to the following displacement reaction. Iron being more reactive than Cu, displaces it thereby forming a new product iron sulphate and copper metal.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
The colour of copper sulphate in test tube A is dark blue and its colour in test tube B becomes greenish due to the formation of FeSO4.
The colour of iron nail becomes brownish when dipped in test tube B as copper metal deposits on it.
Activity 1.10
Answer:
When sodium sulphate reacts with barium chloride, white precipitate of barium sulphate is formed.
![]()
Activity 1.11
The surface of copper powder on heating becomes black. Copper when heated in air reacts with oxygen to produce copper oxide.
Answer:
![]()
Gujarat Board Class 10 Science Chemical Reactions and Equations Textbook Questions and Answers
Question 1.
Which of the following statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Answer:
(i) (a) and (b)
![]()
Question 2.
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction.
Answer:
(d) displacement reaction.
Question 3.
What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer:
(a) Hydrogen gas and iron chloride are produced.
Question 4.
What is balanced chemical equation? Why should chemical equations be balanced?
Answer:
Balanced chemical equations means total number of atoms of each element should be equal on both sides of the reaction in reactants and products. The reaction should be balanced because matter can neither be created nor be destroyed. The total mass of reactants should be equal to total mass of products i.e., mass of reactants = mass of product.
![]()
Question 5.
Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Answer:
(a) 3H2(g) + N2(g) → 2NH3(g)
(b) 2H2S(g) + 3O2(g) → 2H2O(l) + 2SO2(g)
(c) 3BaCl2(aq) + Al2(SO4)3(aq) → 2AlCl3(aq) + 3BaSO4(s)
(d) 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)T
Question 6.
Balance the following chemical equations.
(a) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Answer:
(a) 2HNO3(aq) + Ca(OH)2 (aq) → Ca(NO3)2(aq) + 2H2O(l)
(b) 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l)
(c) NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
(d) BaCl2(aq) H2SO4(aq) → BaSO4(s) + 2HCl(aq)
Question 7.
Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Answer:
(a) Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
(b) Zn(s) + 2AgNO3(aq) → Zn(NO3)2(aq) + 2Ag(s)
(c) 2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu(s)
(d) BaCl2(aq) + K2SO4(aq) → BaSO4(s) + 2KCl(aq)
![]()
Question 8.
Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a) Potassium bromide (aq) + Barium iodide (aq) → Potassium iodide (aq) + Barium bromide (s)
(b) Zinc carbonate (s) → Zinc oxide (s) + Carbon dioxide (g)
(c) Hydrogen (g) + Chlorine (g) → Hydrogen chloride (g)
(d) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride (aq) + Hydrogen (g)
Answer:
(a) 2KBr(aq) + Bal2(aq) → 2KI(aq) + BaBr2(s)
(b) ZnCO3(s) → ZnO(s) + CO2(g)
(c) H2(g) + Cl2(g) → 2HCl(g)
(d) Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Question 9.
What does one mean by exothermic and endothermic reactions? Give examples.
Answer:
Exothermic: Those reactions in which heat is evolved are called exothermic reaction. Heat symbol A is shown at product side.
Example:
C(s) + O2(g) → CO2(g) + ∆
Endothermic: Those reactions in which heat is absorbed are called endothermic reaction. Heat symbol A is shown at reactant side.
Example:
CaCO3(s) + ∆ → CaO(s) + CO2(g)
Question 10.
Why is respiration considered as exothermic reaction? Explain.
Answer:
- In respiration glucose gets oxidized to form carbon dioxide, water and heat is evolved
- As heat energy is released during respiration it is regarded as exothermic reaction.
Question 11.
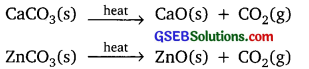
Why are the decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer:
In decomposition reaction, single compound is broken down into simpler compounds or elements.
Example:
CaCO3(s) → CaO(s) + CO2(g)
In combination reaction, two or more elements or compounds combine to form a single new compound.
Example:
CO2(g) + CaO(s) → CaCO3(s)
2SO2(g) + O2(g) → 2SO3(g)
Hence the decomposition and combination are opposite to each other.
![]()
Question 12.
Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity.
Answer:
In decomposition reaction, a compound is broken down into simpler compounds or elements
Example:
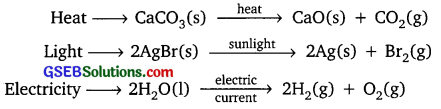
Question 13.
What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer:
In displacement reaction more reactive metal can displace a less reactive metal from its salt solution.
Example:
Zn(s) + CuCl2(aq) → ZnCl2(aq) + Cu(s)
In double displacement reaction, two different compounds exchange their ions and
form new compounds.
Example:
NaOH + HCl → NaCl + H2O
Question 14.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Answer:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Question 15.
What do you mean by precipitation reaction? Explain it by giving examples.
Answer:
Those reactions in which reactants react to form an insoluble compound, i.e. precipitate are called precipitation reactions.
Example:
![]()
Question 16.
Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Answer:
(a) Oxidation: It is a process in which gain of oxygen takes place or loss of hydrogen takes place.
Example:
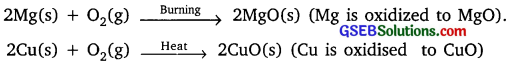
(b) Reduction: It is a process in which loss of oxygen takes place or gain of hydrogen takes place.
Example:
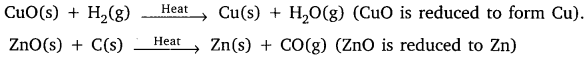
Question 17.
A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Answer:
X is a copper metal, on heating in air it gets oxidized to form CuO which is black in colour.
![]()
Question 18.
Why do we apply paint on iron articles?
Answer:
We apply paint on iron articles so as to prevent it from rusting. When the surface of iron is coated with paint, its surface does not come in contact with oxygen and moisture, therefore, rusting does not take place.
![]()
Question 19.
Oil and fat containing food items are flushed with nitrogen. Why?
Answer:
The food items containing oil and fat are flushed with nitrogen because oil and fat become rancid on oxidation which has the bad taste and smell. The nitrogen flushing prevents the oxidation of food so that it does not become rancid.
Note: (Food is kept in refrigerator so as to reduce the temperature which slows down the rate of oxidation and preserve the food for longer time).
Question 20.
Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Answer:
(a) Corrosion: It is a process in which metal reacts with substances like moisture and gases present in atmosphere to form surface compounds. For example, iron reacts with oxygen in the presence of moisture and forms rust. Silver turns black due to formation of silver sulphide and copper turns green due to the formation of copper carbonate.
(b) Rancidity: It is a process in which food material gets spoiled when it comes in contact with oxygen. It leads to the change in taste and smell of food materials. For example, butter gets spoiled due to oxidation at room temperature if kept for a longer time, it becomes sour in taste and gives a foul smell. The product formed on oxidation of food is rancid and such a process is called rancidity.
Gujarat Board Class 10 Science Chemical Reactions and Equations Additional Important Questions and Answers
Very Short Answer Type Questions
Question 1.
Give the formula for lime.
Answer:
CaO
Question 2.
Give the formula for iron (II) oxide and iron (III) oxide.
Answer:
FeO is iron (II) oxide
Fe2O3 is iron (III) oxide
![]()
Question 3.
Give the formula for rust.
Answer:
Fe2O3.xH2O
Question 4.
Name the conditions necessary for rusting.
Answer:
Oxygen and moisture
Question 5.
Name the reaction seen during rancidity of food.
Answer:
Oxidation
Question 6.
Name the different forms of energy required for breaking down the reactants in decomposition reaction.
Answer:
Heat, light and electricity
Question 7.
What is the insoluble substance formed in a chemical reaction called?
Answer:
Precipitate.
![]()
Question 8.
What does (II) in lead (II) nitrate indicate?
Answer:
It indicates that the valency (oxidation state) of lead in this case is 2 and it form +2 ions.
Question 9.
What are noble metals?
Answer:
Metals which do not react at any temperature, e.g., gold.
Question 10.
Name two metals which do not corrode?
Answer:
Gold and platinum
Question 11.
Name the products obtained when silver bromide is exposed to sunlight.
Answer:
Silver and bromine
Question 12.
Name the gas which burns with pop sound.
Answer:
Hydrogen gas
![]()
Question 13.
Name the compound used to test the evolution of carbon dioxide gas.
Answer:
Calcium hydroxide solution (freshly prepared), also called lime water, is used to test CO2 gas.
Question 14.
Name the gas evolved when lead nitrate is heated.
Answer:
Nitrogen dioxide and oxygen.
Question 15.
Write the formula for two oxides of sulphur.
Answer:
SO2 and SO3
Question 16.
Give the examples of exothermic reaction.
Answer:
Respiration and water added to lime
Question 17.
Give one example of decomposition reaction that occurs in nature.
Answer:
Rotting of fruits and vegetables
Question 18.
Name the type of reaction in which two or more than two reactants form a single compound.
Answer:
Combination reaction
Question 19.
What is breaking and making of bonds in chemicals called?
Answer:
Chemical reaction
Question 20.
Name the chemical used in black and white photography.
Answer:
Silver bromide
Question 21.
Name the ions present in barium sulphate.
Answer:
Ba2+ and SO42-
Question 22.
Name the reaction which forms insoluble salts.
Answer:
Precipitation reaction
![]()
Question 23.
State the reaction in which hydrogen acts as a reducing agent.
Answer:
CuO + H2 → Cu + H2O
Question 24.
Give one use of quick lime.
Answer:
It is used in manufacture of cement.
Question 25.
Give one example of decomposition reaction in which solid and gas are two products obtained.
Answer:
CaCO3 → CaO + CO2
CaO is a solid and carbon dioxide is a gas.
Question 26.
AgNO3(aq) + NaCl(aq) → AgCl(s) ↓+ NaNO3(aq)
FeS + H2SO4 → FeSO4 + H2↑
Consider the above mentioned two chemical equations with two different kinds of arrows (↑ and ↓) along with product. What do these two different arrows indicate?
Answer:
↑ and ↓ shows the gas is evolved whereas ↓ shows insoluble substance (precipitate) is formed.
Short Answer Type Questions
Question 1.
What happens when, carbon dioxide and water react in the same ratio?
Answer:
When 6 molecules of carbon dioxide and 6 molecules of water react together glucose is formed with the evolution of oxygen .gas.
6CO2 + 6H2O → C6H12O6 + 6O2
Question 2.
How can you chemically remove the black coating of copper oxide?
Answer:
The black coating of copper oxide can be removed chemically by passing hydrogen gas over heated copper oxide. The black coating turns brown as oxygen is removed by hydrogen.
Question 3.
Products formed when A and B react together are zinc chloride and hydrogen gas. Find reactants A and B.
Answer:
The reactant A is zinc metal and the reactant B is hydrochloric acid.
Zn + 2HCl → ZnCl2 + H2
Question 4.
Name the products obtained and type of reaction given below:
Na2SO4 + BaCl2 → _______ + _______
Answer:
The products obtained in the above reaction are as follows:
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
The reaction is double displacement.
![]()
Question 5.
When quick lime is added to water a hissing sound is produced. Write the chemical reaction and name the type of reaction taking place.
Answer:
CaO + H2O → Ca(OH)2 + ∆
It is exothermic and combination reaction.
Question 6.
Three test tubes are taken and labelled as A, B and C. In test tube A iron nail is dipped in water. In test tube B iron nail is dipped in mixture of water and oil and in test tube C iron nail is added with dry calcium chloride. Name the test tube in which I the iron nail will rust and why?
Answer:
The iron nail will rust in test tube A because it provides the condition required for ; rusting, both moisture as well as air is present in it.
Question 7.
Metal X becomes green when left in air, turns black when heated in air. Name the metal and the compounds formed in botrfi the cases.
Answer:
X is copper.
Green compound is due to formation of copper carbonate and black colour compound is due to the formation of copper oxide.
Question 8.
Give two examples of a reaction which is both endothermic and decomposition in nature.
Answer:
Question 9.
Explain why most of the metal articles become dull when kept exposed to air?
Answer:
Metal articles react with the gases present in the air and their surface gets coated with the layer of compound it forms making them look dull and the lustre is lost. For example, aluminium metal reacts readily with oxygen to form aluminium oxide and its surface becomes dull.
Question 10.
What is rancidity? What is the general name of chemicals which are added to fat and oil containing food so as to prevent the rancidity?
Answer:
The oil and fat containing food when left exposed to air reacts with oxygen and gets oxidized and become rancid, this process is called rancidity. In this process their smell and taste changes. The general name of the chemicals that are added to prevent I this oxidation are called as antioxidants. For example, nitrogen gas is an antioxidant.
Question 11.
Define displacement reaction. Give one example of it, how is it different from double displacement reaction?
Answer:
The reaction in which more reactive metal displaces (takes place of) the less reactive metal from its compound, is called as displacement reaction,
Example:
Fe(s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
It is different from double displacement reaction because in double displacement reaction two different elements or atoms or ions are exchanged for each other.
Example:
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
![]()
Question 12.
Give differences between the exothermic and endothermic reactions.
Answer:
Exothermic reaction: In this reaction heat is given out during the reaction.
Endothermic reaction: In this reaction heat is taken in to break the bonds and to form new compounds.
Question 13.
Explain and name the type of reaction seen when iron reacts with hydrochloric acid.
Answer:
Iron reacts with HCl to form hydrogen gas and iron chloride. This reaction is called as displacement reaction.
Question 14.
Why is photosynthesis considered as endothermic reaction?
Answer:
Photosynthesis is a reaction in which energy is required to form glucose from carbon dioxide and water. Energy in the form of sunlight is required to break the bonds of , hydrogen and oxygen. Hence it is termed as endothermic reaction.
Question 15.
What is electrolytic decomposition? Give two uses of electrolytic decomposition reaction.
Answer:
When electricity is passed through a molten compound which is ionic in nature, then the ions of the compound separate into its components thereby decomposing the compound. It is used in separating hydrogen and oxygen gas from water. It is also used to decompose sodium chloride to sodium metal and chlorine gas.
Question 16.
Give one example for each of the following reactions:
- Combination reaction
- Decomposition reaction
- Displacement reaction
Answer:
- Combination reaction 2Mg + O2 → 2MgO
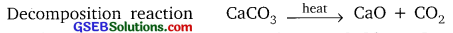
- Displacement reaction Zn + CuSO4 → ZnSO4+ Cu
Question 17.
Give three ways used to prevent rusting.
Answer:
- Oiling of metals
- Applying paints
- Making an alloy
![]()
Question 18.
Define corrosion, rusting and rancidity.
Answer:
Corrosion: Metals when kept exposed, it reacts with moisture, acids and other gases present in atmosphere and get corroded. This process is called corrosion.
Rusting: Iron metal when left exposed, it reacts with moisture and air and get coated with reddish brown substance called rust, this process is called rusting.
Rancidity: Food containing fats and oil when exposed to air gets oxidised and gives bad taste and smell to food. This process is called rancidity.
Question 19.
Name the type of reaction for the following:
- Vegetable matter changing into compost.
- Burning of natural gas.
- Adding water to quick lime to form slaked lime.
Answer:
- Vegetable matter changing into compost is exothermic and decomposition reaction.
- Burning of natural gas is exothermic reaction.
- Adding water to quick lime to form slaked lime is combination reaction and exothermic in nature.
Question 20.
How can one make an equation more informative?
Answer:
To make a chemical equation more informative:
- The physical state of the reactants and products are mentioned like gaseous (g), liquids (l), aqueous (aq), solid (s).
- Conditions such as temperature, pressure catalyst is mentioned above/below the arrow in the equation.
![]()
Question 21.
Give the chemical equations (balanced) for the following:
- Reaction used in black and white photography.
- Reaction when glucose is oxidised.
- Formation of water from H2 and O2.
Answer:
- C6H12O6(S) + 6O2(aq) → 6CO2(g) + 6H2O(g) + Energy
- H2(g) + O2(g) → 2H2O(1)
Question 22.
Give equations to show the chemical reactions of zinc and lead where it displaces copper from its compound.
Answer:
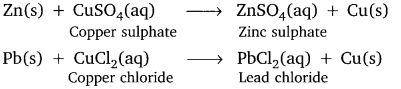
Question 23.
Give an example each for thermal decomposition and photochemical decomposition reactions. Write relevant balanced chemical equations also.
Answer:
Thermal decomposition reaction:

Long Answer Type Questions
Question 1.
Name different types of chemical reactions. Give one example for each.
Answer:
Different types of chemical reactions are:
(a) Combination reactions: The reaction in which two or more than two substances combine together to form a single compound.
Example:
2Mg + O2 → 2 MgO
(b) Decomposition reaction: The reaction in which a compound decomposes to form two or more substances is called decomposition reaction.
![]()
(c) Displacement reaction: The reaction in which more reactive metal displaces the less reactive metal is called displacement reaction.(d) Double displacement reaction: The reaction in which two different atoms or groups of atoms exchange for each other is called double displacement reaction.
Example:
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
(e) Oxidation – reduction reaction: The reaction in which oxygen is added/hydrogen is removed is called oxidation reaction. The reaction in which hydrogen is added/oxygen is removed is called reduction.
Example:
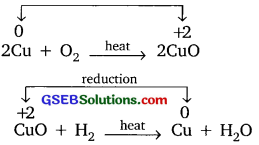
Question 2.
Give an activity to prove that water contains H : O in the ratio of 2 : 1.
Answer:
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in figure.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- The gas collected in test tube attached to cathode is twice in volume than the gas collected at anode.
Once the test tubes are filled with the respective gases, remove them carefully. - The burning match stick or candle when brought near to the test tube containing gas obtained at cathode, burns with a pop sound indicating the presence of hydrogen gas.
Question 3.
What is rancidity? Suggest two methods to reduce the problem of rancidity. How is corrosion different from rusting?
Answer:
1. Rancidity: Fat and oil containing food when kept open in air gets oxidised and become rancid due to which the taste and smell of food changes.
2. Two methods to reduce rancidity are keep the food in closed containers and use antioxidants.
3. Corrosion is seen in all metals, when kept exposed. Forms a layer of compound by reaction of metal with moisture, acid or gases present in it. Rusting is the process in which iron metal reacts with air and moisture to form brownish powder called rust.
![]()
Question 4.
What is meant by exothermic and endothermic reaction? Give examples to explain the same.
Answer:
Exothermic reactions: Those reactions in which heat is released out during chemical reactions.
Example:
![]()
The natural gas (methane) burns in air to form carbon dioxide and water and releases large amount of heat. Endothermic reaction: Those reactions in which heat is absorbed or required, is called endothermic reaction.
![]()
When CaCO3 is heated it forms CaO and CO2.
Question 5.
What is a redox reaction? When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
Answer:
The reaction which shows both oxidation and reduction reaction in it is called redox reaction.
2Mg + O2 → 2MgO
In this case, magnesium is oxidised as oxygen combines with it.
Questions On Higher Order Thinking Skills (Hots)
Question 1.
Name the term used for the solution of the reactant or product when dissolved in water.
Answer:
Aqueous
Question 2.
State one advantage and one disadvantage of corrosion.
Answer:
Advantage: In some metals a protective layer is formed on its surface to prevent it from further corrosion.
Example: aluminium (Al) forms a layer of Aluminium oxide (Al2O3) by corrosion. The layer prevents further corrosion.
Disadvantage: Loss of the metal.
![]()
Question 3.
List four changes which help us to determine whether a chemical reaction has taken place.
Answer:
- Change in state
- Change in colour
- Change in temperature
- Evolution of a gas
Question 4.
Ahmad took a magnesium ribbon (cleaned) and burned it on a flame. The white powder formed was taken in a test tube and water was added to it. He then tested the solution formed with red and blue litmus paper. What change was seen and why?
Answer:
Red litmus turned blue.
Blue litmus remained blue.
This is because the magnesium ribbon on burning in air, forms the white magnesium oxide. When this is dissolved in water, it forms magnesium hydroxide, which is basic in nature.
Question 5.
Give one example of a combination reaction in which an element combines with a compound to give you a new compound.
Answer:
O2 + 2SO2 → 2SO3 or 2Mg + O2 → 2MgO
Question 6.
Deepa added magnesium into a test tube containing dilute hydrochloric acid. She saw some gas coming out of it. She took a burning match stick near the mouth of the test tube and she heard a popping sound while the match stick extinguished. Deepa concluded that the gas evolved is hydrogen and it is not combustible. Find the error in her conclusion and support your answer with one valid reason.
Answer:
The conclusion that hydrogen gas is not combustible is wrong, because hydrogen gas is highly combustible and burns with explosion, to produce large amount of heat. Due to which match stick gets extinguished.
![]()
Question 7.
Arnav took magnesium and reacted it with dil. HCl to record the observation. Then Deepak took the same piece of magnesium and reacted it with cone. HNO3 and dil. H2SO4 but did not see a reaction. Explain this behavior.
Answer:
Arnav had already reacted Mg with dil. HCl to form MgCl2 and gas. The piece of metal that Deepak used was not magnesium but magnesium chloride (MgCl2), hence it did not react with the given acids.
Question 8.
Name the type of reaction seen in the following cases:
- Garbage producing foul smell
- Black and white photograph film when exposed to sunlight
- Carbon dioxide gas passed through lime water
Answer:
- Decomposition reaction
- Decomposition
- Combination.
Question 9.
Four beakers with chemicals are shown below. Name the beakers which will show exothermic reaction and those which will be endothermic in nature.
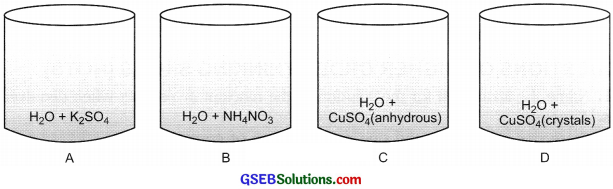
Answer:
Exothermic reaction: Beaker A, Beaker B.
Endothermic reaction: Beaker C, Beaker D.
Question 10.
Give two examples in which reactants react to show combination reaction and a new product is formed. This new product decomposes to give the initial reactants.
Answer:
The reactions in which reactants combine to form products and then again decomposes to give initial reactants are called reversible reactions.
Example:

Question 11.
Do all combination reactions get decomposed to produce the same reactants?
Answer:
No.
Question 12.
Why are certain reagents like silver bromide stored in dark bottles in the labs?
Answer:
Reagents/chemicals like silver bromide decompose when exposed to light. Hence, they are kept in dark bottles in labs, to prevent exposure to light and their decomposition.
![]()
Question 13.
Give six uses of decomposition reaction.
Answer:
- It helps all the living matter to return back to nature, after death.
- Management of garbage
- Photosynthesis → decomposition of water
- Photography → decomposition of silver bromide
- Used in chemical industry to obtain elements from complex compounds.
- Decomposition of agricultural waste leads to formation of compost.
Question 14.
Mohan took pure water for the electrolytic decomposition of water but did not see any bubble near the electrodes. Explain why?
Answer:
Pure water has covalent bond and therefore, does not allow the electricity to flow through it and does not get decomposed. On adding a few drops of dil. acid in it, the free ions are obtained and electricity flows through water to dissociate hydrogen and oxygen.
Question 15.
A teacher took few crystals of sugar in a dry test tube and heated the test tube over flame. The colour of sugar turned black. Explain why?
Answer:
Sugar is a complex compound which on heating undergoes decomposition. Water from sugar gets evaporated thereby leaving behind only black carbon in the test tube.
Question 16.
Blue crystals of copper sulphate on heating in a dry test tube become colourless. Give reasons.
Answer:
The blue colour of copper sulphate is due to its crystalline nature which holds 5 water molecules (water of crystallization). On heating, the water molecules disappear and anhydrous copper sulphate (white in colour) is left back.
![]()
Question 17.
FeSO4.7H2O, green colour crystals on heating, changes colour. Why?
Answer:
The green colour of ferrous sulphate crystals is due to the presence of 7 water molecules (water of crystallization). It loses the water of
crystallization on heating, thus leading to change in colour.
Practical Based Questions (Solved)
Question 1.
Predict what would happen if you add zinc coated iron nail into the solution of copper sulphate. Give reason for your prediction.
Answer:
The zinc metal coating on the iron nail will react with the coper sulphate solution and the blue colour of the solution will slowly fade to become light and after some time the solution will turn green because now the iron nail will react with the copper sulphate. This is because iron and zinc both are more reactive than copper in copper sulphate and the displacement reaction is seen.
Question 2.
How can you differentiate sodium metal and zinc metal given in the test tubes in the lab. You are advised not to touch any of the metals. Name the type of reaction seen.
Answer:
In both of the test tubes I shall add cold water and observe the reaction, the test tube in which the reaction is completed faster will have sodium and the reaction is combination and exothermic in nature.
![]()
Question 3.
A student wants to find how fast the reaction is completed and to do so he places a ‘X’ marked on the tile, above it he places the conical flask with sodium sulphate in it. After some time he adds the solution of barium chloride and records the time taken for the “X’ to disappear. Explain what makes the cross disappear. Name the type of reaction.
Answer:
In this reaction the solutions of barium chloride and sodium sulphate react together to form a white precipitate of barium sulphate which makes the visibility through the new product difficult and such a reaction is called double displacement reaction.
Question 4.
A student wants to study different types of decomposition reactions in the lab. Name the chemicals and the materials required to do this experiment.
Answer:
Chemicals required will be: calcium carbonate for thermal decomposition, silver bromide for photolytic /light decomposition and tap water for electrolytic decomposition.
Materials: beaker, test tubes, burner, tripod stand, pair of tongs, battery, crocodile clips, wires, electrodes of carbon, hand gloves, goggles, lab coat.
![]()
Question 5.
A student wants to study the decomposition reaction of iron sulphate in lab, what precautions should the student take and why?
Answer:
The student should wear lab coat, hand gloves and safety goggles as heating of test tube containing iron sulphate may cause burns. The student should keep the exhaust fan on or perform the heating in the fume cupboard as the gas released on decomposition is sulphur dioxide which has chocking smell. The student can also use the mask or avoid the inhalation of the gas in the lab.
Question 6.
The teacher wants to show that all the crystals in the lab will decompose to give water on heating which is collected on the upper surface of inside of the test tube. Name any four compounds the teacher should use in the lab to perform this activity.
Answer:
The teacher can use, blue vitrol i.e. copper sulphate (blue crystal salt), green vitrol i.e. iron sulphate (green crystal salt), sodium carbonate (white coloured crystals) and calcium sulphate (white coloured crystals).
![]()
Question 7.
Give one example of the combination reaction that is also exothermic in nature and how can you show its exothermic nature in the lab.
Answer:
The reaction of lime, calcium oxide with water is highly exothermic and releases heat which can be measured using thermometer in the lab.
If you come across any doubt related to GSEB Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations, do share your queries in the comment section below. We will surely get back to you.