Gujarat Board GSEB Textbook Solutions Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Textbook Questions and Answers.
Gujarat Board Textbook Solutions Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
GSEB Class 11 Chemistry Some Basic Concepts of Chemistry Text Book Questions and Answers
Question 1.
Calculate the molecular mass of the following :
- H2O
- CO2
- CH4
Solution:
1. Molecular mass of H2O
= 2 × Atomic mass of H + Atomic mass of O = 2 × 1 + 16 = 18.0 a.m.u.
2. Molecular mass of CO2
= Atomic mass of C + 2 × Atomic mass of O = 12 + 2 × 16 = 44.0 a.m.u.
3. Molecular mass of CH4
= Atomic mass of C + 4 × Atomic mass of H = 12 + 4 × 1 = 12 + 4 = 16 a.m.u.
Question 2.
Calculate the mass percent of different elements present in sodium sulfate (Na2SO4).
Solution:
Molecular mass of Na2SO4
= 2 × At. mass of Na + At. mass of S + 4 × At. mass of O = 2 × 23 + 32 + 4 × 16 = 142
![]()
\(\frac { 46 }{ 142 }\) × 100 = 32.39
![]()
\(\frac { 32 }{ 142 }\) × 100 = 22.53
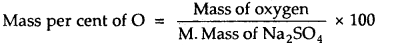
\(\frac { 64 }{ 142 }\) × 100 = 45.07
![]()
Question 3.
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
Solution:
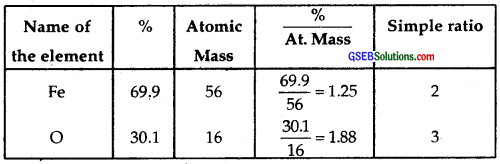
The ratio between Fe and O is 2 : 3.
∴ The empirical formula = Fe2O3.
Question 4.
Calculate the amount of carbon dioxide that could be produced when.
- 1 mole of carbon is burnt in the air
- 1 mole of carbon is burnt in 16 g of dioxygen
- 2 moles of carbon are burnt in 16 g of dioxygen.
Solution:
1.
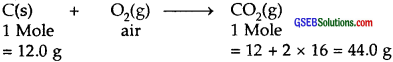
Amount of CO2 produced = 44.0 g
2.
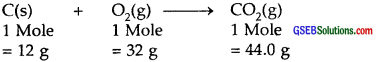
32.0 g of O2 produce 44.0 g of CO2
16.0 g of O2 produce = \(\frac { 44 }{ 32 }\) × 16 = 22.0 g of CO2
Amount of CO2 produced = 22.0 g
3. Amount of CO2 produced when 2 moles (= 24 g) of C are burnt in 16.0 g [limited amount] of O2 = 22.0 g
![]()
Question 5.
Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol-1
Solution:
1000 mL of CH3COONa of 1 M solution contains 82.0245 g
500 mL of it 1 M solution contains = \(\frac { 82.0245 }{ 1000 }\) × 500
500 mL of it of 0.375 M solution = \(\frac { 82.0245 }{ 1000 }\) × 500 × 0.375 = 15.38 g.
Question 6.
Calculate the concentration of nitric acid in moles per liter in a sample that has a density, 1.41 g mL-1 and the mass percent of nitric acid in it being 69%.
Solution:
69% HNO3 by mass means that 69 g of HNO3 is present in 100 g of the solution.
Volume of 100 g of the solution = ![]() = \(\frac { 100 }{ 1.41 }\) = 70.92 mL
= \(\frac { 100 }{ 1.41 }\) = 70.92 mL
Molar mass of HNO3 = 63.0 g mol-1
∴ 69 g HNO3 = \(\frac { 69.0 }{ 63.0 }\) moles = 1.095 moles
Thus 70.92 mL of solution contains HNO3 = 1.095 moles
∴ 1000 mL (= 1 L) solution contains HNO3 = \(\frac { 1.095 }{ 70.92 }\) × 1000
∴ Concentration of nitric acid = 15.44 mol L-1.
![]()
Question 7.
How much copper can be obtained from 100 g of copper sulfate (CuSO4)?
Solution:
Molar mass of CuSO4 = Atomic mass of Cu + Atomic mass of S + 4 × Atomic mass of O
= 63.5 + 32.0 + 4 × 16.0 = 159.5 g mol-1
159.5 g of CuSO4 = 63.5 g of Cu
100 g of CuSO4 = \(\frac { 63.5 }{ 159.5 }\) × 100 = 39.81 g
∴ Mass of Cu that can be obtained = 39.81 g.
Question 8.
Determine the molecular formula of an oxide of iron in which the mass percent of iron and oxygen are 69.9 and 30.1 respectively.
Solution:
Step I: To calculate the empirical formula
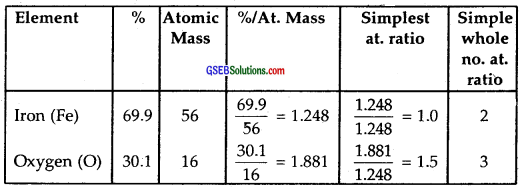
Hence the empirical formula of an oxide of Iron = Fe2O3.
Step II: The molecular formula of the oxide of iron is the same as the empirical formula, i.e., Fe2O3.
Note: Molecular formula can be determined if the molecular mass of oxide of iron is given. It is not given in the question.
![]()
Question 9.
Calculate the atomic mass (average) of chlorine using the following data :
Solution:
The average atomic mass of chlorine![]()
\(\frac { 3545277 }{ 100 }\) = 35.45
Question 10.
In three moles of ethane (C2H6), calculate the following:
- number of moles of carbon atoms
- no. of moles of hydrogen atoms
- no. of molecules of ethane.
Solution:
1. 1 Mole of ethane (C2H6) contains 2 moles of carbon atoms
∴ 3 moles of C2H6 will contain 6 moles of carbon (C) atoms.
2. 1 mole of C2H6 contains 6 moles of hydrogen (H) atoms
∴ 3 moles of C2H6 will contain 3 × 6 = 18 moles of H atoms.
3. 1 mole of C2H6 contains 6.023 × 1023 molecules of ethane
∴ 3 moles of C2H6 will contain 3 × 6.023 × 1023
= 1.8069 × 1024 molecules.
![]()
Question 11.
What is the concentration of sugar (C12H22O11) in mol L-1 if its 20 g are dissolved in enough water to make a final volume up to 2 L?
Solution:
Molar mass of sugar = 12 × 12 + 22 × 1 × 11 × 16 = 342 g mol-1
No. of moles of sugar in 20 g = \(\frac { 20 }{ 342 }\)
Concentration in mol L-1 = No. of moles per litre of the solution
= \(\frac{20}{342 \times 2}\) [∵ Vol. of solution = 2 L]
= 0.029 mol L-1
Question 12.
If the density of methanol is 0.793 kg L-1 h What is its volume needed for making 2.5 L of its 0.25 M solution ?
Solution:
Molar mass of methanol (CH3OH) = 1 × 12 + 4 × 1 + 1 × 16 = 32 g mol-1
Moles of methanol in 2.5 L of its 0.25 M solution = 2.5 × 0.25 = 0.625 mole
mole Mass of methanol = 32 × 0.625 g = 20.0 g
Density of methanol = 0.793 kg L-1
∴ Volume of methanol required = \(\frac { 20.0 }{ 793 }\) – 0.025 L
![]()
Question 13.
The pressure is determined as force per unit area of the surface. The SI unit of pressure, pascal is as shown below :
![]()
If the mass of air at sea level is 1034 g cm-2, calculate the pressure in the pascal.
Solution:
Acceleration due to gravity (g) = 9.806 ms-2
Mass of air = 1.034 kg cm-2 = ![]()
![]()
= 10.138 × 104 Pa
The pressure of air at sea level = 1.0138 × 105 pa.
Question 14.
What is the SI unit of mass? How is it defined?
Solution:
SI unit of mass is Kg (kilogram) It is defined as the mass of platinum-iridium (Pt-Ir) cylinder that is stored in an airtight jar at the International Bureau of Weights and Measures in France.
![]()
Question 15.
Match the following prefixes with their multiples.

Answer:

Question 16.
What do you mean by significant figures?
Solution:
Significant figures refer to the number of digits in a number that has some importance in the magnitude of a given number.
Example: 30.4560
n is equal to 5.
Example: 16708.4300
n is equal to 7.
Question 17.
A sample of drinking water was found to be severely contaminated with chloroform, CHCl3, supposed to be carcinogenic in nature. The level of contamination was 15 ppm (by mass)
- Express this in percent by mass.
- Determine the molality of chloroform in the water sample.
Solution:
1. 106 gm of solution contains 15 g of CHCT3
1 gm of solution contains = ![]()
100 gm of solution contains ![]()
∴ percent by mass = ~ 15 × 10-4g.
2. Molafity of CHCl3 : 106 gm of the solution contains 15 g of CHCl3
∴ Wt. of water 1000000 – 15 = 999985 gm
Now, 999985 g of water contains 15 g of CHCl3
1000 g of water contains = \(\frac { 15 }{ 999985 }\) × 1000 g of CHCl3
Molar mass of CHCl3 = 12 + 1 + 3 × 35.5 = 119.5 g mol-1
∴ Monality = \(\frac { 15 }{ 999985 }\) × \(\frac { 1000 }{ 119.5 }\)m = 1.25 × 10-4m.
![]()
Question 18.
Express the following in the scientific notation
- 0.0048
- 234,000
- 8008
- 500.0
- 6.0012
Solution:
- 0.0048 =\(\frac { 48 }{ 10000 }\)= 48 × 10-4 = 4.8 × 10-3
- 234,000 = 2.34 × 105
- 8008 = 8.008 × 103
- 500.0 = 5.00 × 103
- 6.0012 = 6.0012 × 10°.
Question 19.
How many significant figures are present in the following?
- 0.0025
- 208
- 5005
- 126,000
- 500.0
- 2.0034
Solution:
- 2
- 3
- 4
- 6
- 4
- 5
Question 20.
Round up the following up to three significant figures :
- 34.216
- 10.4107
- 0.04597
- 280.8
Solution:
- 34.126 ~ 34.2
- 10.4107 ~ 10.4
- 0.04597 ~ 0.0460
- 280.8 ~ 281
![]()
Question 21.
The following data are obtained when dinitrogen and dioxygen react together to form different compounds :
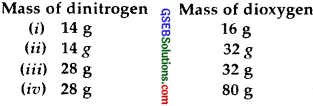
(1) Which law of chemical combination is obeyed by the above experimental data? Give its statement.
(2) Fill in the blanks in the following conversion :
- 1 km = _______ mm = _______ pm
- 1 mg = _______ kg = _______ ng
- 1 mL = _______ L = _______ dm3
Solution:
(1) The given data is in accordance with the law of multiple proportions, which states; When two elements combine to form two or more than two compounds, the weights of one of the two elements which combine with the fixed weight of the order bears a simple ratio to one another.
In the said question, if we fix the weight of dinitrogen at 14 g, then the weights of dioxygen which combines with the fixed weight (=14 g) of dinitrogen will be 16, 32, 16, 40 which are in the simple whole-number ratio of 1: 2: 1: 2.5 or 2: 4: 2: 5.
(2)
- 1 km = 106 mm = 1015 pm
- 1 mg = 10-6 kg = 106 ng
- 1 mL = 10-3 L = 10-3 dm3
Question 22.
If the speed of light is 3.0 × 108 ms-1, calculate the distance covered by light in 2.00 ns.
Solution:
Distance covered by light in one second = 3.0 × 108 m
∴ Distance covered by light in 2.00 ns or 2 × 10-9 second = 3.0 × 108 × 2 × 10-9 m
∴ Distance covered in 2.00 ns = 6.0 × 10-1 m = 0.6 m.
![]()
QUestion 23.
In a reaction, ![]() Identify the limiting reagent, if any, in the following reaction mixtures :
Identify the limiting reagent, if any, in the following reaction mixtures :
- 300 atoms of A + 200 molecules of B
- 2 mol A + 3 mol B
- 100 atoms of A + 100 molecules of B
- 5 mol A + 2.5 mol B
- 2.5 mol A + 5 mol B.
Solution:
- The given reaction is

Here 300-atoms of A requires 300 molecules of B. Since there are only 200 molecules of B provide.
∴ B is the limiting reagent. - 3 mol B requires 2 mol A. Since only 2 mol of A is provided, therefore A is the limiting reagent.
- 100 atoms of A + 100 molecules of B constitute a stoichiometric mixture. Neither A nor B is the limiting reagent.
- B is the limiting reagent as 5 mol A requires 5 mol B but only 2.5 mol B is given.
- A is the limiting reagent as 5 mol B requires 5 mol A, but only 2.5 mol A are provided.
Question 24.
Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation :
![]()
- Calculate the mass of ammonia produced if 2.00 × 103 g dinitrogen reacts with 1.00 × 103 g of dihydrogen.
- Will any of the two reactants remain unreacted?
- If yes, which one and what would be its mass?
Solution:
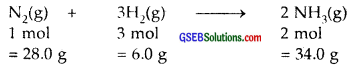
1. 28.0 g of N2 require 6.0 g of H2 to produce 34.0 g of NH3
2.00 × 103 g of N2 will produce \(\frac { 34 }{ 28 }\) × 2.00 × 103 g of NH3
= 2.43 × 103 g NH3 = 2430 g NH3
2. Yes, Dihydrogen will remain unreacted to some extent
3. Amount of hydrogen that remains unreacted 28.0 g of N2 require 6.0 g of H2
200 × 103 g of N2 will require \(\frac { 6.0 }{ 28.0 }\) × 2.00 × 103 of H2 = 428.5 g of H2
∴ Amount of hydrogen = (1.00 × 103 – 428.5) g
that remains unreacted = 571.5 g
![]()
Question 25.
How are 0.50 mol Na2CO3 and 0.50 M Na2CO3 different?
Solution:
0.50 mol Na2CO3 means \(\frac { 1 }{ 2 }\) the molar mass of Na2CO3
![]()
∴ 0.50 mol Na2CO3 represents the mass = 53.0 g of it whereas 0.50 M Na2CO3 represents its molarity in solution 0.50 M Na2CO3 indicates that 53.0 g of Na2CO3 have been dissolved in 1 L of its solution.
Question 26.
If ten volumes of dihydrogen gas react with five volumes of dioxygen gas, how many volumes of water vapor would be produced?
Solution:
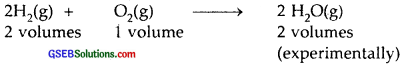
∴ The ratio by volumes is 2: 1: 2
∴ 10 volumes of dihydrogen will react with 5 volumes of dioxygen to produce 10 volumes of water vapors.
Question 27.
Convert the following into basic units :
- 28.7 pm
- 1515 ps
- 25365 mg
Solution:
- 28.7 pm = 2.87 x 10-11 m
- 15.15 ps = 15.15 x 10-6 s ∼ 1.515 x 10-5 s
- 25365 mg = 2.5365 x 10-2 kg.
Question 28.
Which one of the following will have the largest number of atoms?
- 1 g Au(s)
- 1 g Na(s)
- 1 g Li(s)
- 1 g Cl2(g)
Solution:
1. 1 gm atom of Au(s) = 197 g have 6.023 × 1023 atoms of Au
![]()
2. 23.0 g of Na = 6.023 × 1023 atoms of Na
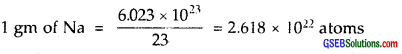
3. 7.0 g of Li = 6.023 × 1023 atoms of Li
![]()
4. 71.0 g of Cl2 = 2 × 6.023 × 1023 atoms of Cl
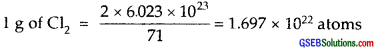
By comparison (iii) i.e., 1 g Li(s) will have max. no. of atoms, viz., 8.604 × 1022 atoms.
Question 29.
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040?
Solution:
Let us calculate the mass of ethanol in which its mole fraction is 0.040.
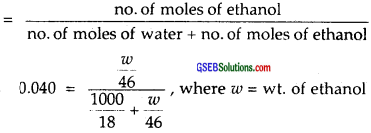
Solving for ω = wt. of ethanol ![]() density is 1 gm cm-3 = 106.48 g
density is 1 gm cm-3 = 106.48 g
∴ Molarity of the solution = \(\frac { 106.48 }{ 46 }\) = 2.315 M.
Question 30.
What will be the mass of one 12C atom in g?
Solution:
We want to calculate the mass of one atom of 12C.
1 gm atom of 12C = 12.0 g
6.23 × 1023 atoms of 12Carbon weigh = 12.0 g
1 atom of 12Carbon weight = ![]()
∴ wt. of 1 atom of 12C = 1.99265 × 10-23 g.
Question 31.
How many significant figures should be present in the answer to the following calculations?
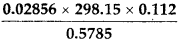
- 5 × 5.364
- 0.0125 + 0.7864 + 0.0215
Solution:
(1) ![]()
It should have 3 significant figures.
(2) 5 × 5.364 = 26.82
It should have 4 significant figures.
(3) 0. 0215 + 0.7864 + 0.0215 = 0.8204
It should have 4 significant figures.
Question 32.
Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes :
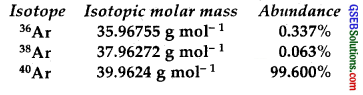
Solution:
The molar mass of naturally occurring Argon

![]()
Question 33.
Calculate the number of atoms in each of the following :
- 52 moles of Ar
- 52 u of He
- 52 g of He.
Solution:
(1) 1 mole of Argon (Ar) contains 6.023 × 1023 atoms
52 moles of Ar contains 6.023 × 1023 × 52 atoms = 3.13 × 1025 atoms
(2) 4 u of Helium (He) = 1 atom of He
52 of He = \(\frac { 1 }{ 4 }\) × 52 = 13 atoms
(3) 4 g of He contains = 6.023 × 1023 atoms
52 g of He contains = ![]() = 7.8286 × 1024 atoms.
= 7.8286 × 1024 atoms.
Question 34.
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water, and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Calculate
- empirical formula
- the molar mass of the gas, and
- molecular formula.
Solution:
Welding fuel gas is made up of C and H only C×Hy. 10.0 L of this gas at STP weighs = 11.6 g
22.4 L of this gas at STP weights = \(\frac { 11.6 }{ 10.0 }\) × 22.4 g = 25.98
Since 22.4 L of any gas at STP weighs = Molecular mass
∴ Molar mass of the welding fuel gas = 26.0 (near to whole no.)
∴ It must contain 2 atoms of C and 2 atoms of hydrogen
∴ Its molecular formula is C2H2 [x = y = 2]
Its empirical formula is CH.
![]()
Question 35.
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction,![]()
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
Solution:
The given reaction is ![]()
Let us find out the weight of HCl present in 25 mL of 0.75 M HCl 1000 mL of 1.0 M HCl contains = 36.5 g of it
1000 mL of 1.0 M HCl contains ![]()
According to the equation;
73 g of HCl [2(1 + 35.5)] reacts with 100.0 g of CaCO3
0.6844 g of HCI reacts with ![]()
Question 36.
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction
![]()
How many grams of HCl react with 5.0 g of manganese dioxide?
Solution:
The chemical equation is
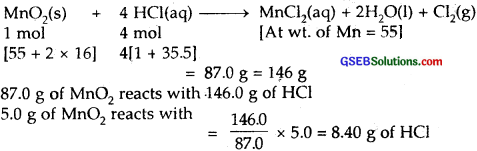
∴ Amount of HCl in grams which will react with 5.0 g of manganese dioxide = 8.40 g.