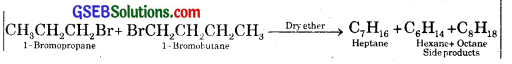Gujarat Board GSEB Textbook Solutions Class 11 Chemistry Chapter 13 Hydrocarbons Textbook Questions and Answers.
Gujarat Board Textbook Solutions Class 11 Chemistry Chapter 13 Hydrocarbons
GSEB Class 11 Chemistry Hydrocarbons Text Book Questions and Answers
![]()
Question 1.
How do you account for the formation of ethane during chlorination of methane?
Answer:
One of the termination step during the formation of chlorination of methane involves the side reaction of the combination of two methyl free radicals (*CH3) as shown below.
.CH3 + .CH3 → H3C:CH3 or C2H6 (ethane)
Question 2.
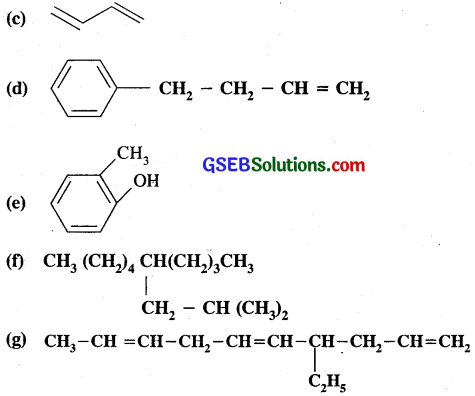
Write IUPAC names of the following compounds:
(a) CH3CH = C(CH3)2
(b) CH2 = CH – C ≡ C – CH3
Answer:
The I.U.P.A.C. names of the compounds are
(a) 2 Methyl-but-2 – ene
(b) Pent-1-ene-3- yne
(c) Buta 1,3 – diene
(d) 4 – Phenyl-but-1- ene
(e) 2 Methylphenol
(f) 5 – (2-Methylpropyl)-decane
(g) 4 Ethyl deca-1, 5, 8 – triene.
![]()
Question 3.
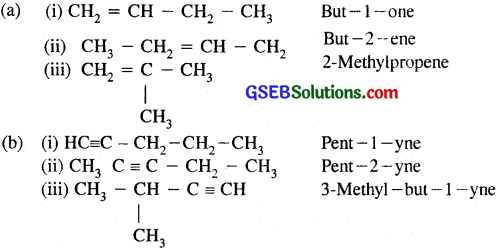
For the following compounds, write structural formulas and IUPAC names for all possible isomers having number of double or triple bonds as indicated:
(a) C4H8 (one double bond)
(b) C5H8 (one triple bond)
Answer:
Question 4.
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) Pent-2-ene
(ii) 3, 4-Dimethylhept-3-ene
(iii) 2-Ethylbut -1-ene
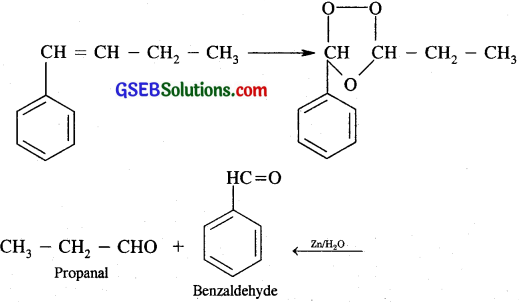
(iv) 1-Phenylbut -1-ene
Answer:
(i) Pent-2-ene is CH3 – CH = CH – CH2 – CH3
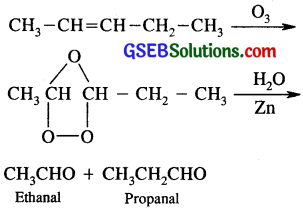
(ii)
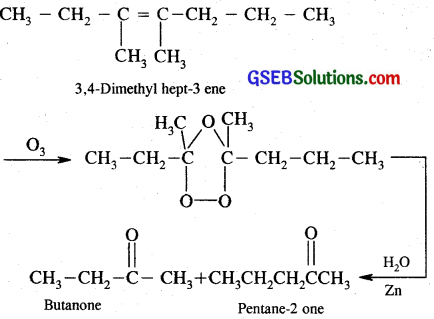
(iii) 2 – Ethyl-but – 1 -ene is
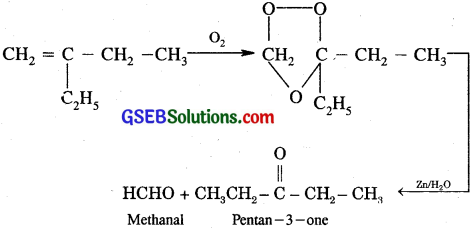
Question 5.
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and 3-Pentanone. Write structure and IUPAC name of ‘A’.
Answer:
Since alkene A on ozonolysis gives a mixture of ethanal and 3-Pentanone, its structure is
Question 6.
An alkene ‘A’ contains three C – C, eight C-Ho bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Answer:
Alkene A contains 3C – C, 8C – H σ bonds and one C – C n bond. Alkene A has one C = C double bond. An aldehyde containing one – CHO group and having molar mass of 44 a.m.u. i, has to be CH3 – CHO and since two moles of CH3CHO are obtained on ozonolysis of alkene A, the alkene has to be joined by two CH3CH groups by a double bond. It has to be CH3 – CH = CH – CH3, i.e., But-2-ene. But-2-enecontains 3C – C σ bonds, 8 C – H σ bonds and one C – C π bond.
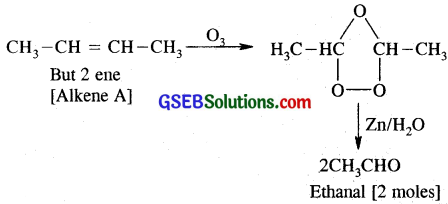
Question 7.
Propanal and pentan -3 -one are the ozonolysis products of an alkene. What is the structural formula of alkene?
Answer:
Since propanal (CH3CH2 CHO) and pentan-3 – one 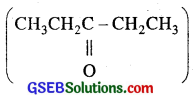 are the ozonolysis product of the alkene, therefore, the alkene is
are the ozonolysis product of the alkene, therefore, the alkene is 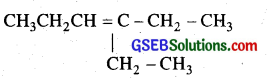 i. e. 4-Ethyl hex-3-ene.
i. e. 4-Ethyl hex-3-ene.
Question 8.
Write chemical equations of combustion reaction of the following by hydrocarbons.
(i) Butane
(ii) Pentene
(iii) Hexyne
(iv) Toluene
Answer:
Butane and all other hydrocarbons on combustion give CO2 and H2O vapours as follows:
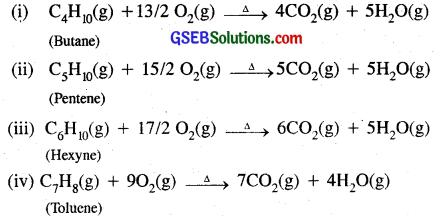
Question 9.
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.pt. and why?
Answer:
Hex-2-ene is CH3-CH2-CH2-CH = CH – CH3. Its CIS and TRANS forms are given below:
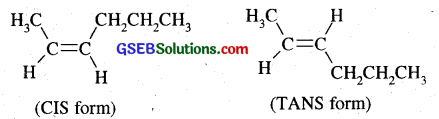
The cis isomer will have higher boiling point due to more polar nature leading to stronger intermolecular dipole-dipole interactions thus requiring more heat energy to separate them, whereas trans form being non-polar (or weakly polar) have weak induced dipole interactions and so have lower boiling point.
![]()
Question 10.
Why is benzene extra ordinarily stable though it contains three double bonds?
Answer:
Benzene (C6H6) has 2 reasonating structures.

Due to resonance between the two above canonical structures benzene is provided with extra stability.
Question 11.
What are the necessary conditions for any system to be aromatic?
Answer:
The necessary conditions for any system to be aromatic are:
- The molecule should be planar
- It should be cyclic with alternate single and double bonds and the cyclic n cloud should surround all the carbon atoms at the ring.
- It should contain (4n + 2)π electrons where n = 0, 1, 2, 3 …. etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
Question 12.
Explain why the following system are not aromatic?
Answer:
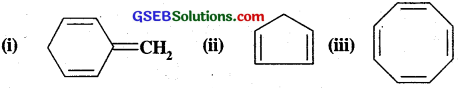
All the three structures lack the cyclic cloud of π-electrons having (4n+2)π electrons.
Question 13.
How will you convert benzene into
(i) p-nitrobromobenzene
(ii) m-nitrochlorobenzene
(iii) p-nitrotoluene
(iv) acetophenone?
Answer:
(i) Benzene into p-nitrobromobenzene:
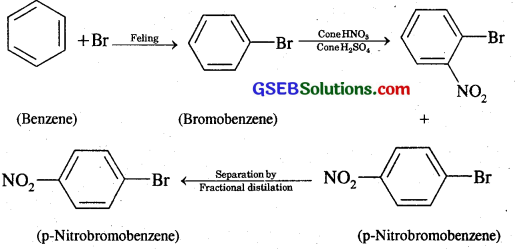
(ii) Benzene into m-Nitroehlorobenzene:
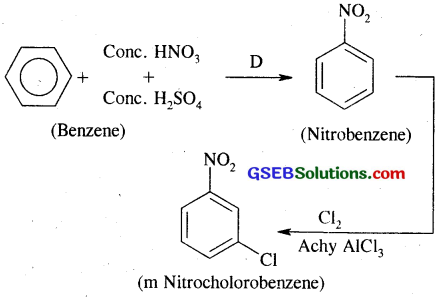
(iii) Benzene into p-nitrotoluene:
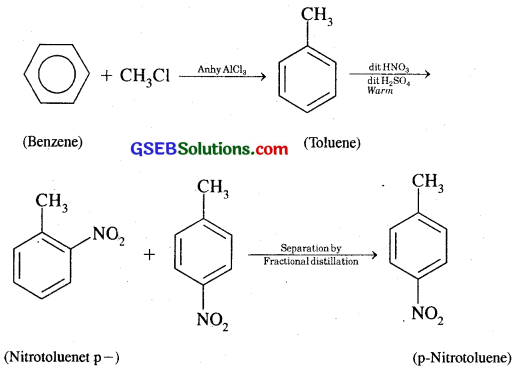
(iv) Benzene into acetophenone:
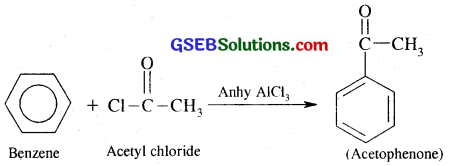
Question 14.
In the alkane H3 C – CH2 – C – (CH3)2 – CH2 – CH (CH3)2, identify 1°, 2°, 3° carbon atoms and give the total number of H atoms bonded to each one of these.
Answer:
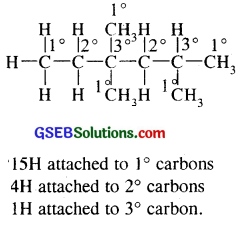
Question 15.
What effect does branching of an alkane chain has on its boiling point?
Answer:
More the branching in alkane, lower will be the boiling point.
![]()
Question 16.
Addition of HBr to propene yields 2-bromo propane, while in presence of benzoyl peroxide the same reaction yields 1- bromopropane. Explain and give mechanism.
Answer:
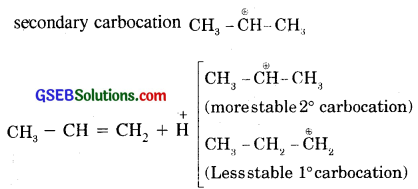
Addition of HBr to propene (unsymmetrical alkene) follows Markovnikov Rule according to which the negative part of the addendum gets attached to that C atom which possesses lesser number of hydrogen atoms. Thus according to this rule 2-Bromopropane is the expected product. In actual practice, it is found as the major product.

Modern Mechanism: Electrophilic Addition.
Step, (i) It follows that proton H+ (an electrophile) from H-Br gets attached to C1 [due to + 1 effect exerted by – CH3group, double bond between C1 and C2 undergoes electromeric effect]
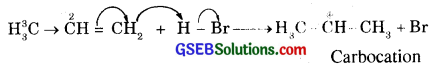
(ii) In the (iii) Step
The intermediate carbocation is attached by Br to form
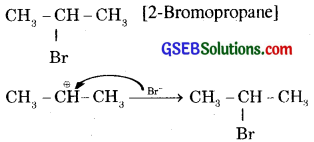
Formation of 2-Bromopropane as the major product and not 1-bromopropane can also be better understood in terms of the stability of the secondary carbocation
Addition of HBr to propene in the presence of benzoyl peroxide follows Anti-markovnikov rule or peroxide effect or kharash effect /Addition of HBr to unsymmetrical alkenes like propene in the presence of light or peroxide takes place contrary in the Markovnikov rule. This so happens only with HBr but not with HC1 and HI. This departure from the rule is known as the peroxide or Kharash effect or anti Markovnikov addition.

Mechanism. Peroxide effect proceeds via free radical mechanism as given below:
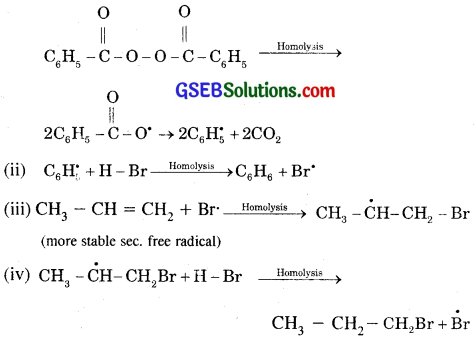
The secondary free radical obtained in (iii) in the above mechanism happens to be more stable than the primary one hence, explains the formation of 1 bromopropane.
Question 17.
Write down the products of ozonolysis 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
Answer:
Ozonolysis of 1, 2-dimethylbenzene (o-xylene) results in the formation of the following products:

All the three products cannot be obtained by any one of the Kekule’s structures. This shows that benezene is a resonance-hybrid , of the two resonating structures.
![]()
Question 18.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for the behaviour.
Answer:
Benzene, n-hexane, and ethyne, in the drcreasing order of acid strength are ethyne > Benzene > n-hexane, i.e., H – C C H > C6H6 > C6H14. Acidic character is based upon the presence of s-character of C – H bond. Due to sp hybridization in ethyne, it is 50% in benzene it is 33% due to sp² hybridization while in n-hexane, it is minimum, i.e. 25% due to sp³ hybridization. More the s-character of C – H bond, more the acidic character.
Question 19.
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer:
Due to the presence of delocalisation of sextet of Pi(π) electrons (6π electrons), benzene behaves as a rich source of electrons [Lewis Base], It is thus readily attacked by electrophiles [reagents deficient in electrons] rather than nucleophiles thus giving electrophilic substitution reaction.
Question 20.
How would you convert the following compounds to benzene?
(i) Ethyne
(ii) Ethene
(iii) Hexane
Answer:
(i) Conversion of ethyne (C2H2) to benzene (C6H6)

(ii) Conversion of ethene to benzene
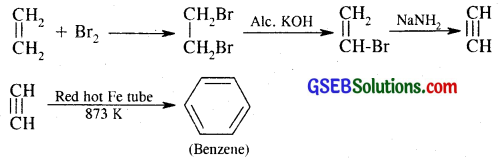
(iii) Conversion of hexane to benzene
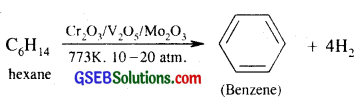
Question 21.
Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer:
Various possible alkenes which on hydrogenation give 2-methylbutane are:
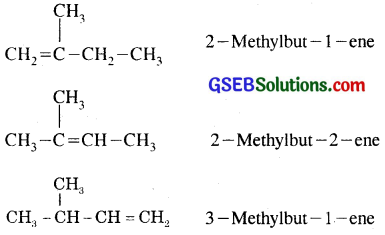
Question 22.
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2, 4 -dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p – H3C – C6H4 – NO2, p- O2N C6H4 – NO2.
Answer:
The order of reactivity towards electrophie E+ in order of their decreasing relative reactivity is –
(a) Chlorobenzene > p-nitrochlorobenzene > 2, 4- dinitrochlorobenzene
(b) Toluene > p – CH3 – C6H4 – NO2 > p – O2NC6H4 – NO2.
![]()
Question 23.
Out pf benzene, m-dinitrobenzene and toluene which will undergo nitration more easily and why?
Answer:
Nitration of benzene is an electrophilic substitution reaction.

NO+2 (nitronium) ion is the attacking electrophile. In toluene, one H of the ring is substituted by – CH3 group which is an electron releasing group and makes the electrons pnore readily available to the electrophile, whereas in m-dinitrobenzene, the two-NO2 groups which are electron – withdrawing in nature makes the availability of electrons of the ring difficult to the electrophile.
Question 24.
Suggest name of another Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene,
Answer:
Ferric chloride (FeCl3).
![]()
Question 25.
Why is Wurtz reaction not preferred for preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Answer:
While preparing higher alkanes by Wurtz reaction only these alkanes are preferably prepared which contain even number of carbon atoms, e.g., ethane, butane, hexane containing respectively 2, 4, 6 carbon atoms.
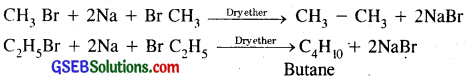
When we prepare propane (C3H8) or pentane (C5H12), there are the chances of the formation of side products, e.g., by starting with l – bromopropane and l – bromobutane, besides the